Novel plant immune-priming compounds identified via high-throughput chemical screening target salicylic acid glucosyltransferases in Arabidopsis
- PMID: 22960909
- PMCID: PMC3480303
- DOI: 10.1105/tpc.112.098343
Novel plant immune-priming compounds identified via high-throughput chemical screening target salicylic acid glucosyltransferases in Arabidopsis
Abstract
Plant activators are compounds, such as analogs of the defense hormone salicylic acid (SA), that protect plants from pathogens by activating the plant immune system. Although some plant activators have been widely used in agriculture, the molecular mechanisms of immune induction are largely unknown. Using a newly established high-throughput screening procedure that screens for compounds that specifically potentiate pathogen-activated cell death in Arabidopsis thaliana cultured suspension cells, we identified five compounds that prime the immune response. These compounds enhanced disease resistance against pathogenic Pseudomonas bacteria in Arabidopsis plants. Pretreatments increased the accumulation of endogenous SA, but reduced its metabolite, SA-O-β-d-glucoside. Inducing compounds inhibited two SA glucosyltransferases (SAGTs) in vitro. Double knockout plants that lack both SAGTs consistently exhibited enhanced disease resistance. Our results demonstrate that manipulation of the active free SA pool via SA-inactivating enzymes can be a useful strategy for fortifying plant disease resistance and may identify useful crop protectants.
Figures
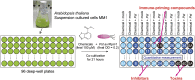
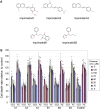



References
-
- Dangl J.L., Jones J.D. (2001). Plant pathogens and integrated defence responses to infection. Nature 411: 826–833 - PubMed
-
- Dean J.V., Mohammed L.A., Fitzpatrick T. (2005). The formation, vacuolar localization, and tonoplast transport of salicylic acid glucose conjugates in tobacco cell suspension cultures. Planta 221: 287–296 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

