Vascular endothelial growth factor-mediated islet hypervascularization and inflammation contribute to progressive reduction of β-cell mass
- PMID: 22961079
- PMCID: PMC3478542
- DOI: 10.2337/db12-0134
Vascular endothelial growth factor-mediated islet hypervascularization and inflammation contribute to progressive reduction of β-cell mass
Abstract
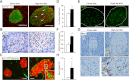
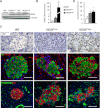
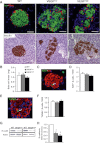
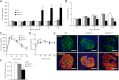
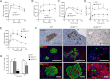
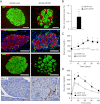
Type 2 diabetes (T2D) results from insulin resistance and inadequate insulin secretion. Insulin resistance initially causes compensatory islet hyperplasia that progresses to islet disorganization and altered vascularization, inflammation, and, finally, decreased functional β-cell mass and hyperglycemia. The precise mechanism(s) underlying β-cell failure remain to be elucidated. In this study, we show that in insulin-resistant high-fat diet-fed mice, the enhanced islet vascularization and inflammation was parallel to an increased expression of vascular endothelial growth factor A (VEGF). To elucidate the role of VEGF in these processes, we have genetically engineered β-cells to overexpress VEGF (in transgenic mice or after adeno-associated viral vector-mediated gene transfer). We found that sustained increases in β-cell VEGF levels led to disorganized, hypervascularized, and fibrotic islets, progressive macrophage infiltration, and proinflammatory cytokine production, including tumor necrosis factor-α and interleukin-1β. This resulted in impaired insulin secretion, decreased β-cell mass, and hyperglycemia with age. These results indicate that sustained VEGF upregulation may participate in the initiation of a process leading to β-cell failure and further suggest that compensatory islet hyperplasia and hypervascularization may contribute to progressive inflammation and β-cell mass loss during T2D.
Figures

References
-
- Mathis D, Vence L, Benoist C. beta-Cell death during progression to diabetes. Nature 2001;414:792–798 - PubMed
-
- Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science 2005;307:380–384 - PubMed
-
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–846 - PubMed
-
- Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia 2002;45:85–96 - PubMed
-
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

