Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma
- PMID: 22961107
- PMCID: PMC3641779
- DOI: 10.1038/nm.2896
Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma
Abstract
Immune tolerance is instituted early in life, during which time regulatory T (T(reg)) cells have an important role. Recurrent infections with respiratory syncytial virus (RSV) in early life increase the risk for asthma in adult life. Repeated infection of infant mice tolerized to ovalbumin (OVA) through their mother's milk with RSV induced allergic airway disease in response to OVA sensitization and challenge, including airway inflammation, hyper-reactivity and higher OVA-specific IgE, as compared to uninfected tolerized control mice. Virus infection induced GATA-3 expression and T helper type 2 (T(H)2) cytokine production in forkhead box P3 (FOXP3)(+) T(reg) cells and compromised the suppressive function of pulmonary T(reg) cells in a manner that was dependent on interleukin-4 receptor α (IL-4Rα) expression in the host. Thus, by promoting a T(H)2-type inflammatory response in the lung, RSV induced a T(H)2-like effector phenotype in T(reg) cells and attenuated tolerance to an unrelated antigen (allergen). Our findings highlight a mechanism by which viral infection targets a host-protective mechanism in early life and increases susceptibility to allergic disease.
Figures
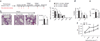
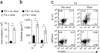



References
-
- Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007;7:379–390. - PubMed
-
- Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am. J. Respir. Crit. Care Med. 2000;161:1501–1507. - PubMed
-
- Sigurs N, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am. J. Respir. Crit. Care Med. 2005;171:137–141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

