MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity
- PMID: 22961109
- PMCID: PMC3745511
- DOI: 10.1038/nm.2899
MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity
Abstract
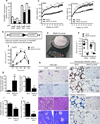
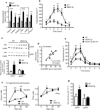
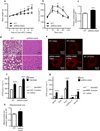
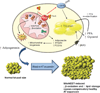
We examined mouse models with altered adipocyte expression of mitoNEET, a protein residing in the mitochondrial outer membrane, to probe its impact on mitochondrial function and subsequent cellular responses. We found that overexpression of mitoNEET enhances lipid uptake and storage, leading to an expansion of the mass of adipose tissue. Despite the resulting massive obesity, benign aspects of adipose tissue expansion prevail, and insulin sensitivity is preserved. Mechanistically, we also found that mitoNEET inhibits mitochondrial iron transport into the matrix and, because iron is a rate-limiting component for electron transport, lowers the rate of β-oxidation. This effect is associated with a lower mitochondrial membrane potential and lower levels of reactive oxygen species-induced damage, along with increased production of adiponectin. Conversely, a reduction in mitoNEET expression enhances mitochondrial respiratory capacity through enhanced iron content in the matrix, ultimately corresponding to less weight gain on a high-fat diet. However, this reduction in mitoNEET expression also causes heightened oxidative stress and glucose intolerance. Thus, manipulation of mitochondrial function by varying mitoNEET expression markedly affects the dynamics of cellular and whole-body lipid homeostasis.
Figures


References
-
- Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401. - PubMed
-
- Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. - PubMed
-
- Mehta JL, Rasouli N, Sinha AK, Molavi B. Oxidative stress in diabetes: a mechanistic overview of its effects on atherogenesis and myocardial dysfunction. Int J Biochem Cell Biol. 2006;38:794–803. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- K99 DK094973/DK/NIDDK NIH HHS/United States
- K99-DK094973/DK/NIDDK NIH HHS/United States
- P01 DK088761/DK/NIDDK NIH HHS/United States
- T32-DK091317/DK/NIDDK NIH HHS/United States
- RC1 DK086629/DK/NIDDK NIH HHS/United States
- R01 DK081842/DK/NIDDK NIH HHS/United States
- R01-DK55758/DK/NIDDK NIH HHS/United States
- T32 DK091317/DK/NIDDK NIH HHS/United States
- RC1-DK086629/DK/NIDDK NIH HHS/United States
- P01-DK088761/DK/NIDDK NIH HHS/United States
- R01-DK081842/DK/NIDDK NIH HHS/United States
- R01 DK055758/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

