Improving FRET dynamic range with bright green and red fluorescent proteins
- PMID: 22961245
- PMCID: PMC3461113
- DOI: 10.1038/nmeth.2171
Improving FRET dynamic range with bright green and red fluorescent proteins
Abstract
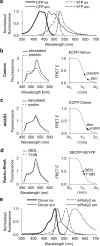
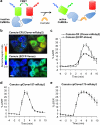
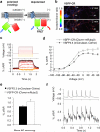
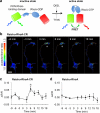
A variety of genetically encoded reporters use changes in fluorescence (or Förster) resonance energy transfer (FRET) to report on biochemical processes in living cells. The standard genetically encoded FRET pair consists of CFPs and YFPs, but many CFP-YFP reporters suffer from low FRET dynamic range, phototoxicity from the CFP excitation light and complex photokinetic events such as reversible photobleaching and photoconversion. We engineered two fluorescent proteins, Clover and mRuby2, which are the brightest green and red fluorescent proteins to date and have the highest Förster radius of any ratiometric FRET pair yet described. Replacement of CFP and YFP with these two proteins in reporters of kinase activity, small GTPase activity and transmembrane voltage significantly improves photostability, FRET dynamic range and emission ratio changes. These improvements enhance detection of transient biochemical events such as neuronal action-potential firing and RhoA activation in growth cones.
Figures
References
-
- Sinnecker D, Voigt P, Hellwig N, Schaefer M. Reversible photobleaching of enhanced green fluorescent proteins. Biochemistry. 2005;44:7085–7094. - PubMed
-
- Raarup MK, et al. Enhanced yellow fluorescent protein photoconversion to a cyan fluorescent protein-like species is sensitive to thermal and diffusion conditions. J Biomed Opt. 2009;14:034039. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

