Revisiting the contribution of negative charges on the chaperonin cage wall to the acceleration of protein folding
- PMID: 22961256
- PMCID: PMC3465421
- DOI: 10.1073/pnas.1204547109
Revisiting the contribution of negative charges on the chaperonin cage wall to the acceleration of protein folding
Abstract
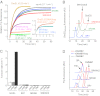
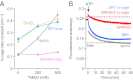
Chaperonin GroEL mediates the folding of protein encapsulated in a GroES-sealed cavity (cage). Recently, a critical role of negative charge clusters on the cage wall in folding acceleration was proposed based on experiments using GroEL single-ring (SR) mutants SR1 and SRKKK2 [Tang YC, et al. (2006) Cell 125:903-914; Chakraborty K, et al. (2010) Cell 142:112-122]. Here, we revisited these experiments and discovered several inconsistencies. (i) SR1 was assumed to bind to GroES stably and to mediate single-round folding in the cage. However, we show that SR1 repeats multiple turnovers of GroES release/binding coupled with ATP hydrolysis. (ii) Although the slow folding observed for a double-mutant of maltose binding protein (DMMBP) by SRKKK2 was attributed to mutations that neutralize negative charges on the cage wall, we found that the majority of DMMBP escape from SRKKK2 and undergo spontaneous folding in the bulk medium. (iii) An osmolyte, trimethylamine N-oxide, was reported to accelerate SRKKK2-mediated folding of DMMBP by mimicking the effect of cage-wall negative charges of WT GroEL and ordering the water structure to promote protein compaction. However, we demonstrate that in-cage folding by SRKKK2 is unaffected by trimethylamine N-oxide. (iv) Although it was reported that SRKKK2 lost the ability to assist the folding of ribulose-1,5-bisphosphate carboxylase/oxygenase, we found that SRKKK2 retains this ability. Our results argue against the role of the negative charges on the cage wall of GroEL in protein folding. Thus, in chaperonin studies, folding kinetics need to be determined from the fraction of the real in-cage folding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science. 2002;295:1852–1858. - PubMed
-
- Horwich AL, Fenton WA. Chaperonin-mediated protein folding: Using a central cavity to kinetically assist polypeptide chain folding. Q Rev Biophys. 2009;42(2):83–116. - PubMed
-
- Weissman JS, et al. Mechanism of GroEL action: Productive release of polypeptide from a sequestered position under GroES. Cell. 1995;83:577–587. - PubMed
-
- Brinker A, et al. Dual function of protein confinement in chaperonin-assisted protein folding. Cell. 2001;107:223–233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

