PTEN deletion enhances survival, neurite outgrowth and function of dopamine neuron grafts to MitoPark mice
- PMID: 22961549
- PMCID: PMC3437026
- DOI: 10.1093/brain/aws196
PTEN deletion enhances survival, neurite outgrowth and function of dopamine neuron grafts to MitoPark mice
Abstract
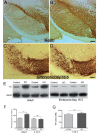
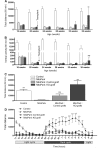
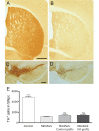
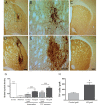
Clinical trials in Parkinson's disease have shown that transplants of embryonic mesencephalic dopamine neurons form new functional connections within the host striatum, but the therapeutic benefits have been highly variable. One obstacle has been poor survival and integration of grafted dopamine neurons. Activation of Akt, a serine/threonine kinase that promotes cell survival and growth, increases the ability of neurons to survive after injury and to regenerate lost neuronal connections. Because the lipid phosphatase, phosphatase and tensin homolog (PTEN) inhibits Akt, we generated a mouse with conditional knock-out of PTEN in dopamine neurons, leading to constitutive expression of Akt in these neurons. Ventral mesencephalic tissue from dopamine phosphatase and tensin homologue knock-out or control animals was then transplanted bilaterally into the dopamine depleted striata of MitoPark mice that express a parkinsonian phenotype because of severe respiratory chain dysfunction in dopamine neurons. After transplantation into MitoPark mice, PTEN-deficient dopamine neurons were less susceptible to cell death, and exhibited a more extensive pattern of fibre outgrowth compared to control grafts. Voltammetric measurements demonstrated that dopamine release and reuptake were significantly increased in the striata of animals receiving dopamine PTEN knock-out transplants. These animals also displayed enhanced spontaneous and drug-induced locomotor activity, relative to control transplanted MitoPark mice. Our results suggest that disinhibition of the Akt-signalling pathway may provide a valuable strategy to enhance survival, function and integration of grafted dopamine neurons within the host striatum and, more generally, to improve survival and integration of different forms of neural grafts.
Figures

Similar articles
-
Non-dopaminergic neurons in ventral mesencephalic transplants make widespread axonal connections in the host brain.Exp Neurol. 2008 Sep;213(1):220-8. doi: 10.1016/j.expneurol.2008.06.005. Epub 2008 Jun 17. Exp Neurol. 2008. PMID: 18602916
-
Transplantation site influences the phenotypic differentiation of dopamine neurons in ventral mesencephalic grafts in Parkinsonian rats.Exp Neurol. 2017 May;291:8-19. doi: 10.1016/j.expneurol.2017.01.010. Epub 2017 Jan 25. Exp Neurol. 2017. PMID: 28131726 Free PMC article.
-
Mesencephalic neural stem (progenitor) cells develop to dopaminergic neurons more strongly in dopamine-depleted striatum than in intact striatum.Exp Neurol. 2000 Jul;164(1):209-14. doi: 10.1006/exnr.2000.7426. Exp Neurol. 2000. PMID: 10877931
-
The time course of loss of dopaminergic neurons and the gliotic reaction surrounding grafts of embryonic mesencephalon to the striatum.Exp Neurol. 1996 Sep;141(1):79-93. doi: 10.1006/exnr.1996.0141. Exp Neurol. 1996. PMID: 8797670 Review.
-
Survival, differentiation, and connectivity of ventral mesencephalic dopamine neurons following transplantation.Prog Brain Res. 2012;200:61-95. doi: 10.1016/B978-0-444-59575-1.00004-1. Prog Brain Res. 2012. PMID: 23195415 Review.
Cited by
-
MiR-92a regulates viability and angiogenesis of endothelial cells under oxidative stress.Biochem Biophys Res Commun. 2014 Apr 18;446(4):952-8. doi: 10.1016/j.bbrc.2014.03.035. Epub 2014 Mar 17. Biochem Biophys Res Commun. 2014. PMID: 24650666 Free PMC article.
-
The Long and the Short of PTEN in the Regulation of Mitophagy.Front Cell Dev Biol. 2020 May 13;8:299. doi: 10.3389/fcell.2020.00299. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32478067 Free PMC article. Review.
-
PTEN inhibition and axon regeneration and neural repair.Neural Regen Res. 2015 Sep;10(9):1363-8. doi: 10.4103/1673-5374.165496. Neural Regen Res. 2015. PMID: 26604880 Free PMC article. Review.
-
The retina as an early biomarker of neurodegeneration in a rotenone-induced model of Parkinson's disease: evidence for a neuroprotective effect of rosiglitazone in the eye and brain.Acta Neuropathol Commun. 2016 Aug 18;4(1):86. doi: 10.1186/s40478-016-0346-z. Acta Neuropathol Commun. 2016. PMID: 27535749 Free PMC article.
-
MicroRNA-29a-3p Regulates SH-SY5Y Cell Proliferation and Neurite Growth through Interaction with PTEN-PI3K/AKT/mTOR Signaling Pathway.Dis Markers. 2022 Aug 1;2022:8151161. doi: 10.1155/2022/8151161. eCollection 2022. Dis Markers. 2022. Retraction in: Dis Markers. 2023 Jul 19;2023:9824132. doi: 10.1155/2023/9824132. PMID: 35958280 Free PMC article. Retracted.
References
-
- Ahn YH, Bensadoun JC, Aebischer P, Zurn AD, Seiger A, Bjorklund A, et al. Increased fiber outgrowth from xeno-transplanted human embryonic dopaminergic neurons with co-implants of polymer-encapsulated genetically modified cells releasing glial cell line-derived neurotrophic factor. Brain Res Bull. 2005;66:135–42. - PubMed
-
- Andereggen L, Meyer M, Guzman R, Ducray AD, Widmer HR. Effects of GDNF pretreatment on function and survival of transplanted fetal ventral mesencephalic cells in the 6-OHDA rat model of Parkinson's disease. Brain Res. 2009;1276:39–49. - PubMed
-
- Antonini A, Schwarz J, Oertel WH, Pogarell O, Leenders KL. Long-term changes of striatal dopamine D2 receptors in patients with Parkinson's disease: a study with positron emission tomography and [11C]raclopride. Mov Disord. 1997;12:33–8. - PubMed
-
- Backman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, et al. Characterization of a mouse strain expressing Cre recombinase from the 3' untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

