The mitochondrial calcium regulator cyclophilin D is an essential component of oestrogen-mediated neuroprotection in amyotrophic lateral sclerosis
- PMID: 22961554
- PMCID: PMC3437032
- DOI: 10.1093/brain/aws208
The mitochondrial calcium regulator cyclophilin D is an essential component of oestrogen-mediated neuroprotection in amyotrophic lateral sclerosis
Abstract
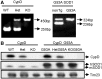
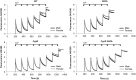
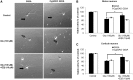
Amyotrophic lateral sclerosis is a devastating neurodegenerative disorder that is more prevalent in males than in females. A similar gender difference has been reported in some strains of transgenic mouse models of familial amyotrophic lateral sclerosis harbouring the G93A mutation in CuZn superoxide dismutase. Mitochondrial damage caused by pathological alterations in Ca(2+) accumulation is frequently involved in neurodegenerative diseases, including CuZn superoxide dismutase-related amyotrophic lateral sclerosis, but its association with gender is not firmly established. In this study, we examined the effects of genetic ablation of cyclophilin D on gender differences in mice expressing G93A mutant CuZn superoxide dismutase. Cyclophilin D is a mitochondrial protein that promotes mitochondrial damage from accumulated Ca(2+). As anticipated, we found that cyclophilin D ablation markedly increased Ca(2+) retention in brain mitochondria of both males and females. Surprisingly, cyclophilin D ablation completely abolished the phenotypic advantage of G93A females, with no effect on disease in males. We also found that the 17β-oestradiol decreased Ca(2+) retention in brain mitochondria, and that cyclophilin D ablation abolished this effect. Furthermore, 17β-oestradiol protected G93A cortical neurons and spinal cord motor neurons against glutamate toxicity, but the protection was lost in neurons lacking cyclophilin D. Taken together, these results identify a novel mechanism of oestrogen-mediated neuroprotection in CuZn superoxide dismutase-related amyotrophic lateral sclerosis, whereby Ca(2+) overload and mitochondrial damage are prevented in a cyclophilin D-dependent manner. Such a protective mechanism may contribute to the lower incidence and later onset of amyotrophic lateral sclerosis, and perhaps other chronic neurodegenerative diseases, in females.
Figures



Similar articles
-
Enhancing mitochondrial calcium buffering capacity reduces aggregation of misfolded SOD1 and motor neuron cell death without extending survival in mouse models of inherited amyotrophic lateral sclerosis.J Neurosci. 2013 Mar 13;33(11):4657-71. doi: 10.1523/JNEUROSCI.1119-12.2013. J Neurosci. 2013. PMID: 23486940 Free PMC article.
-
The mitochondrial permeability transition pore in motor neurons: involvement in the pathobiology of ALS mice.Exp Neurol. 2009 Aug;218(2):333-46. doi: 10.1016/j.expneurol.2009.02.015. Epub 2009 Mar 9. Exp Neurol. 2009. PMID: 19272377 Free PMC article.
-
Calcium dysregulation, mitochondrial pathology and protein aggregation in a culture model of amyotrophic lateral sclerosis: mechanistic relationship and differential sensitivity to intervention.Neurobiol Dis. 2011 Jun;42(3):265-75. doi: 10.1016/j.nbd.2011.01.016. Epub 2011 Feb 3. Neurobiol Dis. 2011. PMID: 21296666
-
Mitochondria in motor nerve terminals: function in health and in mutant superoxide dismutase 1 mouse models of familial ALS.J Bioenerg Biomembr. 2011 Dec;43(6):581-6. doi: 10.1007/s10863-011-9392-1. J Bioenerg Biomembr. 2011. PMID: 22089637 Free PMC article. Review.
-
Transgenic mice with human mutant genes causing Parkinson's disease and amyotrophic lateral sclerosis provide common insight into mechanisms of motor neuron selective vulnerability to degeneration.Rev Neurosci. 2007;18(2):115-36. doi: 10.1515/revneuro.2007.18.2.115. Rev Neurosci. 2007. PMID: 17593875 Review.
Cited by
-
Estrogen receptor beta modulates permeability transition in brain mitochondria.Biochim Biophys Acta Bioenerg. 2018 Jun;1859(6):423-433. doi: 10.1016/j.bbabio.2018.03.006. Epub 2018 Mar 14. Biochim Biophys Acta Bioenerg. 2018. PMID: 29550215 Free PMC article.
-
Mitochondrial Dysfunction in Schizophrenia: Determination of Mitochondrial Respiratory Activity in a Two-Hit Mouse Model.J Mol Neurosci. 2016 Aug;59(4):440-51. doi: 10.1007/s12031-016-0746-3. Epub 2016 Mar 31. J Mol Neurosci. 2016. PMID: 27034067
-
Genetic induction of hypometabolism by ablation of MC4R does not suppress ALS-like phenotypes in the G93A mutant SOD1 mouse model.Sci Rep. 2017 Oct 13;7(1):13150. doi: 10.1038/s41598-017-13304-4. Sci Rep. 2017. PMID: 29030576 Free PMC article.
-
Boosting Mitochondrial Potential: An Imperative Therapeutic Intervention in Amyotrophic Lateral Sclerosis.Curr Neuropharmacol. 2023;21(5):1117-1138. doi: 10.2174/1570159X20666220915092703. Curr Neuropharmacol. 2023. PMID: 36111770 Free PMC article. Review.
-
Mitochondrial dynamic abnormalities in amyotrophic lateral sclerosis.Transl Neurodegener. 2015 Jul 29;4:14. doi: 10.1186/s40035-015-0037-x. eCollection 2015. Transl Neurodegener. 2015. PMID: 26225210 Free PMC article.
References
-
- Alvarez-Delgado C, Mendoza-Rodriguez CA, Picazo O, Cerbon M. Different expression of alpha and beta mitochondrial estrogen receptors in the aging rat brain: interaction with respiratory complex V. Exp Gerontol. 2010;45:580–5. - PubMed
-
- Arieli Y, Gursahani H, Eaton MM, Hernandez LA, Schaefer S. Gender modulation of Ca(2+) uptake in cardiac mitochondria. J Mol Cell Cardiol. 2004;37:507–13. - PubMed
-
- Batra SC. Effect of some estrogens and progesterone on calcium uptake and calcium release by myometrial mitochondria. Biochem Pharmacol. 1973;22:803–9. - PubMed
-
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–55. - PubMed
-
- Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem. 2003;278:19062–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

