Insulin regimens and clinical outcomes in a type 1 diabetes cohort: the SEARCH for Diabetes in Youth study
- PMID: 22961571
- PMCID: PMC3526205
- DOI: 10.2337/dc12-0720
Insulin regimens and clinical outcomes in a type 1 diabetes cohort: the SEARCH for Diabetes in Youth study
Abstract
Objective: To examine the patterns and associations of insulin regimens and change in regimens with clinical outcomes in a diverse population of children with recently diagnosed type 1 diabetes.
Research design and methods: The study sample consisted of youth with type 1 diabetes who completed a baseline SEARCH for Diabetes in Youth study visit after being newly diagnosed and at least one follow-up visit. Demographic, diabetes self-management, physical, and laboratory measures were collected at study visits. Insulin regimens and change in regimen compared with the initial visit were categorized as more intensive (MI), no change (NC), or less intensive (LI). We examined relationships between insulin regimens, change in regimen, and outcomes including A1C and fasting C-peptide.
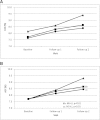
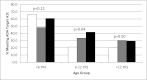
Results: Of the 1,606 participants with a mean follow-up of 36 months, 51.7% changed to an MI regimen, 44.7% had NC, and 3.6% changed to an LI regimen. Participants who were younger, non-Hispanic white, and from families of higher income and parental education and who had private health insurance were more likely to be in MI or NC groups. Those in MI and NC groups had lower baseline A1C (P = 0.028) and smaller increase in A1C over time than LI (P < 0.01). Younger age, continuous subcutaneous insulin pump therapy, and change to MI were associated with higher probability of achieving target A1C levels.
Conclusions: Insulin regimens were intensified over time in over half of participants but varied by sociodemographic domains. As more intensive regimens were associated with better outcomes, early intensification of management may improve outcomes in all children with diabetes. Although intensification of insulin regimen is preferred, choice of insulin regimen must be individualized based on the child and family's ability to comply with the prescribed plan.
Figures

References
-
- Rubin RR, Peyrot M. Implications of the DCCT. Looking beyond tight control. Diabetes Care 1994;17:235–236 - PubMed
-
- Cleary PA, Orchard TJ, Genuth S, et al. DCCT/EDIC Research Group The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes 2006;55:3556–3565 - PMC - PubMed
-
- Silverstein J, Klingensmith G, Copeland K, et al. American Diabetes Association Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care 2005;28:186–212 - PubMed
-
- de Beaufort CE, Swift PG, Skinner CT, et al. Hvidoere Study Group on Childhood Diabetes 2005 Continuing stability of center differences in pediatric diabetes care: do advances in diabetes treatment improve outcome? The Hvidoere Study Group on Childhood Diabetes. Diabetes Care 2007;30:2245–2250 - PubMed
-
- Haller MJ, Silverstein JH. In pursuit of lower A1c. J Pediatr 2009;155:161–162 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DP000247/DP/NCCDPHP CDC HHS/United States
- U01 DP000245/DP/NCCDPHP CDC HHS/United States
- U48/CCU419249/PHS HHS/United States
- M01 RR000069/RR/NCRR NIH HHS/United States
- U18DP002708-01/DP/NCCDPHP CDC HHS/United States
- U18 DP002714/DP/NCCDPHP CDC HHS/United States
- U01 DP000248/DP/NCCDPHP CDC HHS/United States
- U01 DP000244/DP/NCCDPHP CDC HHS/United States
- P30 DK57516/DK/NIDDK NIH HHS/United States
- U48/CCU519239/PHS HHS/United States
- UL1 RR029882/RR/NCRR NIH HHS/United States
- UL1 TR000077/TR/NCATS NIH HHS/United States
- U01 DP000250/DP/NCCDPHP CDC HHS/United States
- P30 DK057516/DK/NIDDK NIH HHS/United States
- HIR 10-001/HX/HSRD VA/United States
- 1U18DP002709/DP/NCCDPHP CDC HHS/United States
- U48/CCU819241-3/PHS HHS/United States
- M01RR00037/RR/NCRR NIH HHS/United States
- U18 DP002710/DP/NCCDPHP CDC HHS/United States
- U18DP002714/DP/NCCDPHP CDC HHS/United States
- U48/CCU919219/PHS HHS/United States
- M01 RR000037/RR/NCRR NIH HHS/United States
- U58CCU919256/PHS HHS/United States
- 1UL1RR026314-01/RR/NCRR NIH HHS/United States
- U18DP000247-06A1/DP/NCCDPHP CDC HHS/United States
- U18DP002710-01/DP/NCCDPHP CDC HHS/United States
- M01RR00069/RR/NCRR NIH HHS/United States
- U58/CCU019235-4/PHS HHS/United States
- U18 DP002709/DP/NCCDPHP CDC HHS/United States
- U01 DP000246/DP/NCCDPHP CDC HHS/United States
- U01 DP000254/DP/NCCDPHP CDC HHS/United States
- UL1 RR026314/RR/NCRR NIH HHS/United States
- U18 DP002708/DP/NCCDPHP CDC HHS/United States
- UL1RR029882/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

