Intrahepatic cholestasis of pregnancy levels of sulfated progesterone metabolites inhibit farnesoid X receptor resulting in a cholestatic phenotype
- PMID: 22961653
- PMCID: PMC3592994
- DOI: 10.1002/hep.26055
Intrahepatic cholestasis of pregnancy levels of sulfated progesterone metabolites inhibit farnesoid X receptor resulting in a cholestatic phenotype
Abstract
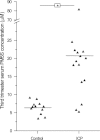
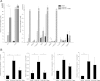
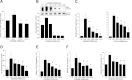
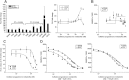
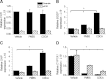
Intrahepatic cholestasis of pregnancy (ICP) is the most prevalent pregnancy-specific liver disease and is associated with an increased risk of adverse fetal outcomes, including preterm labor and intrauterine death. The endocrine signals that cause cholestasis are not known but 3α-sulfated progesterone metabolites have been shown to be elevated in ICP, leading us to study the impact of sulfated progesterone metabolites on farnesoid X receptor (FXR)-mediated bile acid homeostasis pathways. Here we report that the 3β-sulfated progesterone metabolite epiallopregnanolone sulfate is supraphysiologically raised in the serum of ICP patients. Mice challenged with cholic acid developed hypercholanemia and a hepatic gene expression profile indicative of FXR activation. However, coadministration of epiallopregnanolone sulfate with cholic acid exacerbated the hypercholanemia and resulted in aberrant gene expression profiles for hepatic bile acid-responsive genes consistent with cholestasis. We demonstrate that levels of epiallopregnanolone sulfate found in ICP can function as a partial agonist for FXR, resulting in the aberrant expression of bile acid homeostasis genes in hepatoma cell lines and primary human hepatocytes. Furthermore, epiallopregnanolone sulfate inhibition of FXR results in reduced FXR-mediated bile acid efflux and secreted FGF19. Using cofactor recruitment assays, we show that epiallopregnanolone sulfate competitively inhibits bile acid-mediated recruitment of cofactor motifs to the FXR-ligand binding domain.
Conclusion: Our results reveal a novel molecular interaction between ICP-associated levels of the 3β-sulfated progesterone metabolite epiallopregnanolone sulfate and FXR that couples the endocrine component of pregnancy in ICP to abnormal bile acid homeostasis.
Copyright © 2012 American Association for the Study of Liver Diseases.
Figures

References
-
- Fisk NM, Storey GN. Fetal outcome in obstetric cholestasis. Br J Obstet Gynaecol. 1988;95:1137–1143. - PubMed
-
- Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. HEPATOLOGY. 2004;40:467–474. - PubMed
-
- Van Mil SW, Milona A, Dixon PH, Mullenbach R, Geenes VL, Chambers J, et al. Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology. 2007;133:507–516. - PubMed
-
- Pauli-Magnus C, Lang T, Meier Y, Zodan-Marin T, Jung D, Breymann C, et al. Sequence analysis of bile salt export pump (ABCB11) and multidrug resistance p-glycoprotein 3 (ABCB4, MDR3) in patients with intrahepatic cholestasis of pregnancy. Pharmacogenetics. 2004;14:91–102. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
