Using ligand-mapping simulations to design a ligand selectively targeting a cryptic surface pocket of polo-like kinase 1
- PMID: 22961729
- PMCID: PMC3547296
- DOI: 10.1002/anie.201205676
Using ligand-mapping simulations to design a ligand selectively targeting a cryptic surface pocket of polo-like kinase 1
Figures
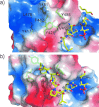
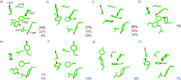
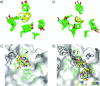

References
-
- Fuentes G, Dastidar SG, Madhumalar A, Verma CS. Drug Dev. Res. 2011;72:26–35.
-
- Betts MJ, Sternberg MJE. Protein Eng. 1999;12:271–283. - PubMed
-
- Withers IM, Mazanetz MP, Wang H, Fischer PM, Laughton CA. J. Chem. Inf. Model. 2008;48:1448–1454. - PubMed
-
- DeLano WL, Ultsch MH, de Vos AM, Wells JA. Science. 2000;287:1279–1283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

