Expanding the concepts and tools of metabolic engineering to elucidate cancer metabolism
- PMID: 22961737
- PMCID: PMC3586222
- DOI: 10.1002/btpr.1629
Expanding the concepts and tools of metabolic engineering to elucidate cancer metabolism
Abstract
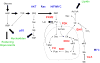
The metabolic engineer's toolbox, comprising stable isotope tracers, flux estimation and analysis, pathway identification, and pathway kinetics and regulation, among other techniques, has long been used to elucidate and quantify pathways primarily in the context of engineering microbes for producing small molecules of interest. Recently, these tools are increasingly finding use in cancer biology due to their unparalleled capacity for quantifying intracellular metabolism of mammalian cells. Here, we review basic concepts that are used to derive useful insights about the metabolism of tumor cells, along with a number of illustrative examples highlighting the fundamental contributions of these methods to elucidating cancer cell metabolism. This area presents unique opportunities for metabolic engineering to expand its portfolio of applications into the realm of cancer biology and help develop new cancer therapies based on a new class of metabolically derived targets.
Copyright © 2012 American Institute of Chemical Engineers (AIChE).
Figures





References
-
- Stephanopoulos G. Metabolic fluxes and metabolic engineering. Metab Eng. 1999;1(1):1–11. - PubMed
-
- Wiechert W. 13C metabolic flux analysis. Metab Eng. 2001;3:195–206. - PubMed
-
- Wiechert W, de Graaf aa. In vivo stationary flux analysis by 13C labeling experiments. Adv Biochem Eng Biotechnol. 1996;54:109–54. - PubMed
-
- Zamboni N, Fendt SM, Rühl M, Sauer U. (13)C-based metabolic flux analysis. Nat Protoc. 2009;4(6):878–92. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

