A conserved UDP-glucose dehydrogenase encoded outside the hasABC operon contributes to capsule biogenesis in group A Streptococcus
- PMID: 22961854
- PMCID: PMC3486382
- DOI: 10.1128/JB.01317-12
A conserved UDP-glucose dehydrogenase encoded outside the hasABC operon contributes to capsule biogenesis in group A Streptococcus
Abstract
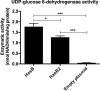
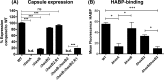
Group A Streptococcus (GAS) is a human-specific bacterial pathogen responsible for serious morbidity and mortality worldwide. The hyaluronic acid (HA) capsule of GAS is a major virulence factor, contributing to bloodstream survival through resistance to neutrophil and antimicrobial peptide killing and to in vivo pathogenicity. Capsule biosynthesis has been exclusively attributed to the ubiquitous hasABC hyaluronan synthase operon, which is highly conserved across GAS serotypes. Previous reports indicate that hasA, encoding hyaluronan synthase, and hasB, encoding UDP-glucose 6-dehydrogenase, are essential for capsule production in GAS. Here, we report that precise allelic exchange mutagenesis of hasB in GAS strain 5448, a representative of the globally disseminated M1T1 serotype, did not abolish HA capsule synthesis. In silico whole-genome screening identified a putative HasB paralog, designated HasB2, with 45% amino acid identity to HasB at a distant location in the GAS chromosome. In vitro enzymatic assays demonstrated that recombinant HasB2 is a functional UDP-glucose 6-dehydrogenase enzyme. Mutagenesis of hasB2 alone slightly decreased capsule abundance; however, a ΔhasB ΔhasB2 double mutant became completely acapsular. We conclude that HasB is not essential for M1T1 GAS capsule biogenesis due to the presence of a newly identified HasB paralog, HasB2, which most likely resulted from gene duplication. The identification of redundant UDP-glucose 6-dehydrogenases underscores the importance of HA capsule expression for M1T1 GAS pathogenicity and survival in the human host.
Figures


Similar articles
-
Molecular characterization of hasB from an operon required for hyaluronic acid synthesis in group A streptococci. Demonstration of UDP-glucose dehydrogenase activity.J Biol Chem. 1993 Apr 5;268(10):7118-24. J Biol Chem. 1993. PMID: 8463246
-
Molecular analysis of the capsule gene region of group A Streptococcus: the hasAB genes are sufficient for capsule expression.J Bacteriol. 1998 Sep;180(18):4955-9. doi: 10.1128/JB.180.18.4955-4959.1998. J Bacteriol. 1998. PMID: 9733702 Free PMC article.
-
Hyaluronic acid synthesis operon (has) expression in group A streptococci.J Biol Chem. 1995 Aug 4;270(31):18452-8. doi: 10.1074/jbc.270.31.18452. J Biol Chem. 1995. PMID: 7629171
-
Human disease isolates of serotype m4 and m22 group a streptococcus lack genes required for hyaluronic acid capsule biosynthesis.mBio. 2012 Nov 6;3(6):e00413-12. doi: 10.1128/mBio.00413-12. mBio. 2012. PMID: 23131832 Free PMC article. Review.
-
The Emergence of Hypervirulent M1T1 Clone of Group A Streptococcus via Genetic Recombination and Host Selection.Curr Issues Mol Biol. 2019;32:435-472. doi: 10.21775/cimb.032.435. Epub 2019 Jun 5. Curr Issues Mol Biol. 2019. PMID: 31166177 Review.
Cited by
-
Capsular Polysaccharide of Group A Streptococcus.Microbiol Spectr. 2019 Jan;7(1):10.1128/microbiolspec.gpp3-0050-2018. doi: 10.1128/microbiolspec.GPP3-0050-2018. Microbiol Spectr. 2019. PMID: 30632480 Free PMC article.
-
The intricate pathogenicity of Group A Streptococcus: A comprehensive update.Virulence. 2024 Dec;15(1):2412745. doi: 10.1080/21505594.2024.2412745. Epub 2024 Nov 5. Virulence. 2024. PMID: 39370779 Free PMC article. Review.
-
Tailor-made exopolysaccharides-CRISPR-Cas9 mediated genome editing in Paenibacillus polymyxa.Synth Biol (Oxf). 2017 Dec 21;2(1):ysx007. doi: 10.1093/synbio/ysx007. eCollection 2017 Jan. Synth Biol (Oxf). 2017. PMID: 32995508 Free PMC article.
-
The Emergence of Successful Streptococcus pyogenes Lineages through Convergent Pathways of Capsule Loss and Recombination Directing High Toxin Expression.mBio. 2019 Dec 10;10(6):e02521-19. doi: 10.1128/mBio.02521-19. mBio. 2019. PMID: 31822586 Free PMC article.
-
Disease manifestations and pathogenic mechanisms of Group A Streptococcus.Clin Microbiol Rev. 2014 Apr;27(2):264-301. doi: 10.1128/CMR.00101-13. Clin Microbiol Rev. 2014. PMID: 24696436 Free PMC article. Review.
References
-
- Alberti S, Ashbaugh CD, Wessels MR. 1998. Structure of the has operon promoter and regulation of hyaluronic acid capsule expression in group A Streptococcus. Mol. Microbiol. 28:343–353 - PubMed
-
- Aziz RK, et al. 2010. Microevolution of group A streptococci in vivo: capturing regulatory networks engaged in sociomicrobiology, niche adaptation, and hypervirulence. PLoS One 5:e9798 doi:10.1371/journal.pone.0009798 - DOI - PMC - PubMed
-
- Bernish B, van de Rijn I. 1999. Characterization of a two-component system in Streptococcus pyogenes which is involved in regulation of hyaluronic acid production. J. Biol. Chem. 274:4786–4793 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

