A novel component of the Rhodobacter sphaeroides Fla1 flagellum is essential for motor rotation
- PMID: 22961858
- PMCID: PMC3486363
- DOI: 10.1128/JB.00850-12
A novel component of the Rhodobacter sphaeroides Fla1 flagellum is essential for motor rotation
Abstract
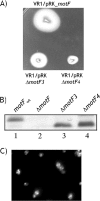
Here we describe a novel component essential for flagellar rotation in Rhodobacter sphaeroides. This protein is encoded by motF (RSP_0067), the first gene of a predicted transcriptional unit which contains two hypothetical genes. Sequence analysis indicated that MotF is a bitopic membrane-spanning protein. Protease sensitivity assays and green fluorescent protein (GFP) fusions confirmed this prediction and allowed us to conclude that the C terminus of MotF is located in the periplasmic space. Wild-type cells expressing a functional GFP-MotF fusion show a single fluorescent focus per cell. The localization of this protein in different genetic backgrounds allowed us to determine that normal localization of MotF depends on the presence of FliL and MotB. Characterization of a ΔmotF pseudorevertant strain revealed that a single nucleotide change in motB suppresses the Mot(-) phenotype of the motF mutant. Additionally, we show that MotF also becomes dispensable when other mutant alleles of motB previously isolated as second-site suppressors of ΔfliL were expressed in the motF mutant strain. These results show that MotF is a new component of the Fla1 flagellum, which together with FliL is required to promote flagellar rotation, possibly through MotB.
Figures

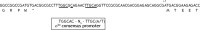



Similar articles
-
Swimming Using a Unidirectionally Rotating, Single Stopping Flagellum in the Alpha Proteobacterium Rhodobacter sphaeroides.Front Microbiol. 2022 Jun 1;13:893524. doi: 10.3389/fmicb.2022.893524. eCollection 2022. Front Microbiol. 2022. PMID: 35722353 Free PMC article. Review.
-
Living in a Foster Home: The Single Subpolar Flagellum Fla1 of Rhodobacter sphaeroides.Biomolecules. 2020 May 16;10(5):774. doi: 10.3390/biom10050774. Biomolecules. 2020. PMID: 32429424 Free PMC article. Review.
-
A distant homologue of the FlgT protein interacts with MotB and FliL and is essential for flagellar rotation in Rhodobacter sphaeroides.J Bacteriol. 2013 Dec;195(23):5285-96. doi: 10.1128/JB.00760-13. Epub 2013 Sep 20. J Bacteriol. 2013. PMID: 24056105 Free PMC article.
-
FliL and its paralog MotF have distinct roles in the stator activity of the Sinorhizobium meliloti flagellar motor.Mol Microbiol. 2022 Sep;118(3):223-243. doi: 10.1111/mmi.14964. Epub 2022 Jul 28. Mol Microbiol. 2022. PMID: 35808893 Free PMC article.
-
The flagellar protein FliL is essential for swimming in Rhodobacter sphaeroides.J Bacteriol. 2010 Dec;192(23):6230-9. doi: 10.1128/JB.00655-10. Epub 2010 Oct 1. J Bacteriol. 2010. PMID: 20889747 Free PMC article.
Cited by
-
Structural analysis of variant of Helicobacter pylori MotB in its activated form, engineered as chimera of MotB and leucine zipper.Sci Rep. 2017 Oct 18;7(1):13435. doi: 10.1038/s41598-017-13421-0. Sci Rep. 2017. PMID: 29044185 Free PMC article.
-
Swimming Using a Unidirectionally Rotating, Single Stopping Flagellum in the Alpha Proteobacterium Rhodobacter sphaeroides.Front Microbiol. 2022 Jun 1;13:893524. doi: 10.3389/fmicb.2022.893524. eCollection 2022. Front Microbiol. 2022. PMID: 35722353 Free PMC article. Review.
-
Living in a Foster Home: The Single Subpolar Flagellum Fla1 of Rhodobacter sphaeroides.Biomolecules. 2020 May 16;10(5):774. doi: 10.3390/biom10050774. Biomolecules. 2020. PMID: 32429424 Free PMC article. Review.
-
Characterization of FlgP, an Essential Protein for Flagellar Assembly in Rhodobacter sphaeroides.J Bacteriol. 2019 Feb 11;201(5):e00752-18. doi: 10.1128/JB.00752-18. Print 2019 Mar 1. J Bacteriol. 2019. PMID: 30559113 Free PMC article.
-
Twists and turns: 40 years of investigating how and why bacteria swim.Microbiology (Reading). 2024 Feb;170(2):001432. doi: 10.1099/mic.0.001432. Microbiology (Reading). 2024. PMID: 38363121 Free PMC article. Review.
References
-
- Armitage JP, Evans MCW. 1985. Control of the protonmotive force in Rhodopseudomonas sphaeroides in the light and dark and its effect on the initiation of flagellar rotation. Biochim. Biophys. Acta 806:42–55
-
- Ausubel FM, et al. 1987. Current protocols in molecular biology, vol 1 John Wiley & Sons, Inc., New York, NY
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

