Role of the Helicobacter pylori-induced inflammatory response in the development of gastric cancer
- PMID: 22961880
- PMCID: PMC3909030
- DOI: 10.1002/jcb.24389
Role of the Helicobacter pylori-induced inflammatory response in the development of gastric cancer
Abstract
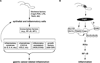
Helicobacter pylori (H. pylori) infection causes chronic gastritis and peptic ulceration and is the strongest risk factor for the development of gastric cancer. The pathogenesis of H. pylori is believed to be associated with infection-initiated chronic gastritis, which is characterized by enhanced expression of many inflammatory genes. H. pylori utilizes various virulence factors, targeting different cellular proteins, to modulate the host inflammatory response. In this review, we explore the many different ways by which H. pylori initiates inflammation, leveling many "hits" on the gastric mucosa which can lead to the development of cancer. We also discuss some recent findings in understanding the pathogen-host interactions and the role of transcription factor NF-κB in H. pylori-induced inflammation.
Copyright © 2012 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no conflict of interest
Figures
Similar articles
-
Helicobacter pylori-induced REDD1 modulates Th17 cell responses that contribute to gastritis.Clin Sci (Lond). 2021 Nov 26;135(22):2541-2558. doi: 10.1042/CS20210753. Clin Sci (Lond). 2021. PMID: 34730176
-
Helicobacter pylori-induced gastric inflammation and gastric cancer.Cancer Lett. 2014 Apr 10;345(2):196-202. doi: 10.1016/j.canlet.2013.08.016. Epub 2013 Aug 24. Cancer Lett. 2014. PMID: 23981572 Review.
-
Nucleotide-binding oligomerization domain 1 and Helicobacter pylori infection: A review.World J Gastroenterol. 2018 Apr 28;24(16):1725-1733. doi: 10.3748/wjg.v24.i16.1725. World J Gastroenterol. 2018. PMID: 29713127 Free PMC article. Review.
-
MicroRNA Modulation of Host Immune Response and Inflammation Triggered by Helicobacter pylori.Int J Mol Sci. 2021 Jan 30;22(3):1406. doi: 10.3390/ijms22031406. Int J Mol Sci. 2021. PMID: 33573346 Free PMC article. Review.
-
Molecular response of gastric epithelial cells to Helicobacter pylori-induced cell damage.Cell Microbiol. 1999 Sep;1(2):93-9. doi: 10.1046/j.1462-5822.1999.00018.x. Cell Microbiol. 1999. PMID: 11207544 Review.
Cited by
-
Gli1 deletion prevents Helicobacter-induced gastric metaplasia and expansion of myeloid cell subsets.PLoS One. 2013;8(3):e58935. doi: 10.1371/journal.pone.0058935. Epub 2013 Mar 8. PLoS One. 2013. PMID: 23520544 Free PMC article.
-
The role of chronic inflammation in obesity-associated cancers.ISRN Oncol. 2013 May 30;2013:697521. doi: 10.1155/2013/697521. Print 2013. ISRN Oncol. 2013. PMID: 23819063 Free PMC article.
-
The effect of Helicobacter pylori infection and different H. pylori components on the proliferation and apoptosis of gastric epithelial cells and fibroblasts.PLoS One. 2019 Aug 7;14(8):e0220636. doi: 10.1371/journal.pone.0220636. eCollection 2019. PLoS One. 2019. PMID: 31390383 Free PMC article.
-
Helicobacter pylori glycan biosynthesis modulates host immune cell recognition and response.Front Cell Infect Microbiol. 2024 Mar 20;14:1377077. doi: 10.3389/fcimb.2024.1377077. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38572314 Free PMC article.
-
Cytokines, cytokine gene polymorphisms and Helicobacter pylori infection: friend or foe?World J Gastroenterol. 2014 May 14;20(18):5235-43. doi: 10.3748/wjg.v20.i18.5235. World J Gastroenterol. 2014. PMID: 24833853 Free PMC article. Review.
References
-
- Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–3226. - PubMed
-
- Allison CC, Kufer TA, Kremmer E, Kaparakis M, Ferrero RL. Helicobacter pylori Induces MAPK phosphorylation and AP-1 activation via a NOD1-dependent mechanism. J Immunol. 2009;183:8099–8109. - PubMed
-
- Backert S, Naumann M. What a disorder: proinflammatory signaling pathways induced by Helicobacter pylori. Trends in Microbiology. 2010;18:479–486. - PubMed
-
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

