Fe(III) reduction and U(VI) immobilization by Paenibacillus sp. strain 300A, isolated from Hanford 300A subsurface sediments
- PMID: 22961903
- PMCID: PMC3485948
- DOI: 10.1128/AEM.01844-12
Fe(III) reduction and U(VI) immobilization by Paenibacillus sp. strain 300A, isolated from Hanford 300A subsurface sediments
Abstract
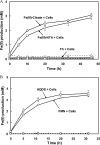
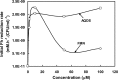
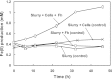
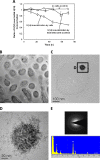
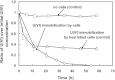
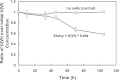
A facultative iron-reducing [Fe(III)-reducing] Paenibacillus sp. strain was isolated from Hanford 300A subsurface sediment biofilms that was capable of reducing soluble Fe(III) complexes [Fe(III)-nitrilotriacetic acid and Fe(III)-citrate] but unable to reduce poorly crystalline ferrihydrite (Fh). However, Paenibacillus sp. 300A was capable of reducing Fh in the presence of low concentrations (2 μM) of either of the electron transfer mediators (ETMs) flavin mononucleotide (FMN) or anthraquinone-2,6-disulfonate (AQDS). Maximum initial Fh reduction rates were observed at catalytic concentrations (<10 μM) of either FMN or AQDS. Higher FMN concentrations inhibited Fh reduction, while increased AQDS concentrations did not. We also found that Paenibacillus sp. 300A could reduce Fh in the presence of natural ETMs from Hanford 300A subsurface sediments. In the absence of ETMs, Paenibacillus sp. 300A was capable of immobilizing U(VI) through both reduction and adsorption. The relative contributions of adsorption and microbial reduction to U(VI) removal from the aqueous phase were ∼7:3 in PIPES [piperazine-N,N'-bis(2-ethanesulfonic acid)] and ∼1:4 in bicarbonate buffer. Our study demonstrated that Paenibacillus sp. 300A catalyzes Fe(III) reduction and U(VI) immobilization and that these reactions benefit from externally added or naturally existing ETMs in 300A subsurface sediments.
Figures
Similar articles
-
Functional diversity and electron donor dependence of microbial populations capable of U(VI) reduction in radionuclide-contaminated subsurface sediments.Appl Environ Microbiol. 2008 May;74(10):3159-70. doi: 10.1128/AEM.02881-07. Epub 2008 Mar 31. Appl Environ Microbiol. 2008. PMID: 18378664 Free PMC article.
-
Enumeration and characterization of iron(III)-reducing microbial communities from acidic subsurface sediments contaminated with uranium(VI).Appl Environ Microbiol. 2003 Dec;69(12):7467-79. doi: 10.1128/AEM.69.12.7467-7479.2003. Appl Environ Microbiol. 2003. PMID: 14660400 Free PMC article.
-
Geobacter daltonii sp. nov., an Fe(III)- and uranium(VI)-reducing bacterium isolated from a shallow subsurface exposed to mixed heavy metal and hydrocarbon contamination.Int J Syst Evol Microbiol. 2010 Mar;60(Pt 3):546-553. doi: 10.1099/ijs.0.010843-0. Epub 2009 Aug 4. Int J Syst Evol Microbiol. 2010. PMID: 19654355
-
Dissimilatory reduction of Fe(III) and other electron acceptors by a Thermus isolate.Appl Environ Microbiol. 1999 Mar;65(3):1214-21. doi: 10.1128/AEM.65.3.1214-1221.1999. Appl Environ Microbiol. 1999. PMID: 10049886 Free PMC article.
-
Microbial iron redox cycling in a circumneutral-pH groundwater seep.Appl Environ Microbiol. 2009 Jan;75(2):468-73. doi: 10.1128/AEM.01817-08. Epub 2008 Dec 1. Appl Environ Microbiol. 2009. PMID: 19047399 Free PMC article.
Cited by
-
Visualizing and Isolating Iron-Reducing Microorganisms at the Single-Cell Level.Appl Environ Microbiol. 2021 Jan 15;87(3):e02192-20. doi: 10.1128/AEM.02192-20. Print 2021 Jan 15. Appl Environ Microbiol. 2021. PMID: 33158896 Free PMC article.
-
A bibliography study of Shewanella oneidensis biofilm.FEMS Microbiol Ecol. 2023 Oct 17;99(11):fiad124. doi: 10.1093/femsec/fiad124. FEMS Microbiol Ecol. 2023. PMID: 37796898 Free PMC article.
-
Effect of Mineral Carriers on Biofilm Formation and Nitrogen Removal Activity by an Indigenous Anammox Community from Cold Groundwater Ecosystem Alone and Bioaugmented with Biomass from a "Warm" Anammox Reactor.Biology (Basel). 2022 Sep 29;11(10):1421. doi: 10.3390/biology11101421. Biology (Basel). 2022. PMID: 36290325 Free PMC article.
-
Impact of Organic Carbon Electron Donors on Microbial Community Development under Iron- and Sulfate-Reducing Conditions.PLoS One. 2016 Jan 22;11(1):e0146689. doi: 10.1371/journal.pone.0146689. eCollection 2016. PLoS One. 2016. PMID: 26800443 Free PMC article.
-
ToF-SIMS spectral data analysis of Paenibacillus sp. 300A biofilms and planktonic cells.Data Brief. 2025 Jun 9;61:111763. doi: 10.1016/j.dib.2025.111763. eCollection 2025 Aug. Data Brief. 2025. PMID: 40605852 Free PMC article.
References
-
- Ahmed B, et al. 2012. Immobilization of U(VI) from oxic groundwater by Hanford 300 Area sediments and effects of Columbia River water. Water Res. 46: 3989– 3998 - PubMed
-
- Ash C, Priest FG, Collins MD. 1993. Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test. Antonie Van Leeuwenhoek 64: 253– 260 - PubMed
-
- Barnett MO, Jardine PM, Brooks SC. 2002. U(VI) adsorption to heterogeneous subsurface media: application of a surface complexation model. Environ. Sci. Technol. 36: 937– 942 - PubMed
-
- Behrends T, Van Cappellen P. 2005. Competition between enzymatic and abiotic reduction of uranium(VI) under iron reducing conditions. Chem. Geol. 220: 315– 327
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

