Snail represses the splicing regulator epithelial splicing regulatory protein 1 to promote epithelial-mesenchymal transition
- PMID: 22961986
- PMCID: PMC3476309
- DOI: 10.1074/jbc.M112.397125
Snail represses the splicing regulator epithelial splicing regulatory protein 1 to promote epithelial-mesenchymal transition
Abstract
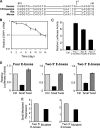
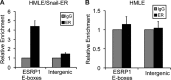
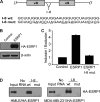
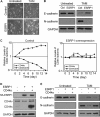
Epithelial-mesenchymal transition (EMT), a tightly regulated process that is critical for development, is frequently re-activated during cancer metastasis and recurrence. We reported previously that CD44 isoform switching is critical for EMT and showed that the splicing factor ESRP1 inhibits CD44 isoform switching during EMT. However, the mechanism by which ESRP1 is regulated during EMT has not been fully understood. Here we show that the transcription repressor Snail binds to E-boxes in the ESRP1 promoter, causing repression of the ESRP1 gene. Biochemically, we define the mechanism by which ESRP1 regulates CD44 alternative splicing: ESRP1 binds to the intronic region flanking a CD44 variable exon and causes increased variable exon inclusion. We further show that ectopically expressing ESRP1 inhibits Snail-induced EMT, suggesting that down-regulation of ESRP1 is required for function by Snail in EMT. Together, these data reveal how the transcription factor Snail mediates EMT through regulation of a splicing factor.
Figures

References
-
- Moody S. E., Perez D., Pan T. C., Sarkisian C. J., Portocarrero C. P., Sterner C. J., Notorfrancesco K. L., Cardiff R. D., Chodosh L. A. (2005) The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell 8, 197–209 - PubMed
-
- Thiery J. P., Sleeman J. P. (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7, 131–142 - PubMed
-
- Yang J., Mani S. A., Donaher J. L., Ramaswamy S., Itzykson R. A., Come C., Savagner P., Gitelman I., Richardson A., Weinberg R. A. (2004) Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939 - PubMed
-
- Yang J., Weinberg R. A. (2008) Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell 14, 818–829 - PubMed
-
- Batlle E., Sancho E., Francí C., Domínguez D., Monfar M., Baulida J., García De Herreros A. (2000) The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumor cells. Nat. Cell Biol. 2, 84–89 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

