The design and characterization of receptor-selective APRIL variants
- PMID: 22961987
- PMCID: PMC3481339
- DOI: 10.1074/jbc.M112.406090
The design and characterization of receptor-selective APRIL variants
Abstract
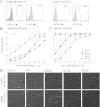
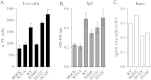
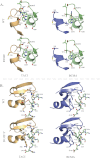
A proliferation-inducing ligand (APRIL), a member of the TNF ligand superfamily with an important role in humoral immunity, is also implicated in several cancers as a prosurvival factor. APRIL binds two different TNF receptors, B cell maturation antigen (BCMA) and transmembrane activator and cylclophilin ligand interactor (TACI), and also interacts independently with heparan sulfate proteoglycans. Because APRIL shares binding of the TNF receptors with B cell activation factor, separating the precise signaling pathways activated by either ligand in a given context has proven quite difficult. In this study, we have used the protein design algorithm FoldX to successfully generate a BCMA-specific variant of APRIL, APRIL-R206E, and two TACI-selective variants, D132F and D132Y. These APRIL variants show selective activity toward their receptors in several in vitro assays. Moreover, we have used these ligands to show that BCMA and TACI have a distinct role in APRIL-induced B cell stimulation. We conclude that these ligands are useful tools for studying APRIL biology in the context of individual receptor activation.
Figures



References
-
- Kimberley F. C., Medema J. P., Hahne M. (2009) APRIL in B-cell malignancies and autoimmunity. Results Probl. Cell Differ. 49, 161–182 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

