Gene duplication and the evolution of hemoglobin isoform differentiation in birds
- PMID: 22962007
- PMCID: PMC3488042
- DOI: 10.1074/jbc.M112.375600
Gene duplication and the evolution of hemoglobin isoform differentiation in birds
Abstract
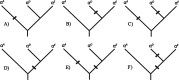
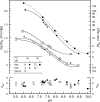
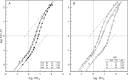
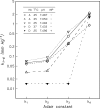
The majority of bird species co-express two functionally distinct hemoglobin (Hb) isoforms in definitive erythrocytes as follows: HbA (the major adult Hb isoform, with α-chain subunits encoded by the α(A)-globin gene) and HbD (the minor adult Hb isoform, with α-chain subunits encoded by the α(D)-globin gene). The α(D)-globin gene originated via tandem duplication of an embryonic α-like globin gene in the stem lineage of tetrapod vertebrates, which suggests the possibility that functional differentiation between the HbA and HbD isoforms may be attributable to a retained ancestral character state in HbD that harkens back to a primordial, embryonic function. To investigate this possibility, we conducted a combined analysis of protein biochemistry and sequence evolution to characterize the structural and functional basis of Hb isoform differentiation in birds. Functional experiments involving purified HbA and HbD isoforms from 11 different bird species revealed that HbD is characterized by a consistently higher O(2) affinity in the presence of allosteric effectors such as organic phosphates and Cl(-) ions. In the case of both HbA and HbD, analyses of oxygenation properties under the two-state Monod-Wyman-Changeux allosteric model revealed that the pH dependence of Hb-O(2) affinity stems primarily from changes in the O(2) association constant of deoxy (T-state)-Hb. Ancestral sequence reconstructions revealed that the amino acid substitutions that distinguish the adult-expressed Hb isoforms are not attributable to the retention of an ancestral (pre-duplication) character state in the α(D)-globin gene that is shared with the embryonic α-like globin gene.
Figures

Similar articles
-
Gene turnover in the avian globin gene families and evolutionary changes in hemoglobin isoform expression.Mol Biol Evol. 2015 Apr;32(4):871-87. doi: 10.1093/molbev/msu341. Epub 2014 Dec 9. Mol Biol Evol. 2015. PMID: 25502940 Free PMC article.
-
Oxygenation properties and isoform diversity of snake hemoglobins.Am J Physiol Regul Integr Comp Physiol. 2015 Nov 1;309(9):R1178-91. doi: 10.1152/ajpregu.00327.2015. Epub 2015 Sep 9. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 26354849 Free PMC article.
-
Probing the conformation of hemoglobin presbyterian in the R-state.J Protein Chem. 2003 Apr;22(3):221-30. doi: 10.1023/a:1025080801951. J Protein Chem. 2003. PMID: 12962322
-
Gene duplication, genome duplication, and the functional diversification of vertebrate globins.Mol Phylogenet Evol. 2013 Feb;66(2):469-78. doi: 10.1016/j.ympev.2012.07.013. Epub 2012 Jul 27. Mol Phylogenet Evol. 2013. PMID: 22846683 Free PMC article. Review.
-
Modulation of Allosteric Control and Evolution of Hemoglobin.Biomolecules. 2023 Mar 22;13(3):572. doi: 10.3390/biom13030572. Biomolecules. 2023. PMID: 36979507 Free PMC article. Review.
Cited by
-
Allosteric mechanisms underlying the adaptive increase in hemoglobin-oxygen affinity of the bar-headed goose.J Exp Biol. 2018 Sep 17;221(Pt 18):jeb185470. doi: 10.1242/jeb.185470. J Exp Biol. 2018. PMID: 30026237 Free PMC article.
-
Gene Turnover and Diversification of the α- and β-Globin Gene Families in Sauropsid Vertebrates.Genome Biol Evol. 2018 Jan 1;10(1):344-358. doi: 10.1093/gbe/evy001. Genome Biol Evol. 2018. PMID: 29340581 Free PMC article.
-
Top-down-assisted bottom-up method for homologous protein sequencing: hemoglobin from 33 bird species.J Am Soc Mass Spectrom. 2015 Nov;26(11):1875-84. doi: 10.1007/s13361-015-1185-z. Epub 2015 Jun 26. J Am Soc Mass Spectrom. 2015. PMID: 26111519 Free PMC article.
-
Changes in Hemoglobin Isoforms in the Peripheral Blood of Rats with Experimental Posthemorrhagic Anemia.Bull Exp Biol Med. 2021 Aug;171(4):421-424. doi: 10.1007/s10517-021-05241-0. Epub 2021 Sep 20. Bull Exp Biol Med. 2021. PMID: 34542743
-
Stability-Mediated Epistasis Restricts Accessible Mutational Pathways in the Functional Evolution of Avian Hemoglobin.Mol Biol Evol. 2017 May 1;34(5):1240-1251. doi: 10.1093/molbev/msx085. Mol Biol Evol. 2017. PMID: 28201714 Free PMC article.
References
-
- Dickerson R. E., Geis I. (1983) Hemoglobin: Structure, Function, Evolution, and Pathology, Benjamin/Cummings Publishing, Menlo Park, CA
-
- Perutz M. F. (1983) Species adaptation in a protein molecule. Mol. Biol. Evol. 1, 1–28 - PubMed
-
- Bunn H. F., Forget B. G. (1986) Hemoglobin: Molecular, Genetic, and Clinical Aspects, W.B. Saunders Co., Philadelphia
-
- Weber R. E., Fago A. (2004) Functional adaptation and its molecular basis in vertebrate hemoglobins, neuroglobins, and cytoglobins. Respir. Physiol. Neurobiol. 144, 141–159 - PubMed
-
- Weber R. E. (1990) in Animal Nutrition and Transport Processes (Truchot J. P., Lahlou B., eds) pp. 58–75, S. Karger AG, Basel, Switzerland
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

