Maternal high-altitude hypoxia and suppression of ryanodine receptor-mediated Ca2+ sparks in fetal sheep pulmonary arterial myocytes
- PMID: 22962012
- PMCID: PMC3517681
- DOI: 10.1152/ajplung.00009.2012
Maternal high-altitude hypoxia and suppression of ryanodine receptor-mediated Ca2+ sparks in fetal sheep pulmonary arterial myocytes
Abstract
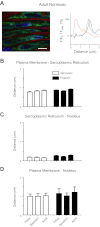
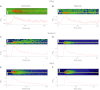
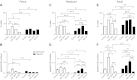
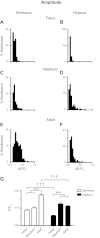
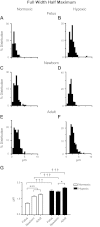
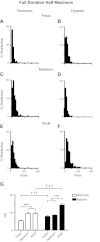
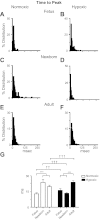
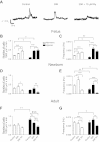
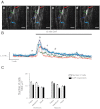
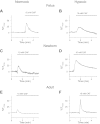
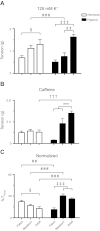
Ca(2+) sparks are fundamental Ca(2+) signaling events arising from ryanodine receptor (RyR) activation, events that relate to contractile and dilatory events in the pulmonary vasculature. Recent studies demonstrate that long-term hypoxia (LTH) can affect pulmonary arterial reactivity in fetal, newborn, and adult animals. Because RyRs are important to pulmonary vascular reactivity and reactivity changes with ontogeny and LTH we tested the hypothesis that RyR-generated Ca(2+) signals are more active before birth and that LTH suppresses these responses. We examined these hypotheses by performing confocal imaging of myocytes in living arteries and by performing wire myography studies. Pulmonary arteries (PA) were isolated from fetal, newborn, or adult sheep that lived at low altitude or from those that were acclimatized to 3,801 m for > 100 days. Confocal imaging demonstrated preservation of the distance between the sarcoplasmic reticulum, nucleus, and plasma membrane in PA myocytes. Maturation increased global Ca(2+) waves and Ca(2+) spark activity, with sparks becoming larger, wider, and slower. LTH preferentially depressed Ca(2+) spark activity in immature pulmonary arterial myocytes, and these sparks were smaller, wider, and slower. LTH also suppressed caffeine-elicited contraction in fetal PA but augmented contraction in the newborn and adult. The influence of both ontogeny and LTH on RyR-dependent cell excitability shed new light on the therapeutic potential of these channels for the treatment of pulmonary vascular disease in newborns as well as adults.
Figures
Similar articles
-
Long-term high-altitude hypoxia influences pulmonary arterial L-type calcium channel-mediated Ca2+ signals and contraction in fetal and adult sheep.Am J Physiol Regul Integr Comp Physiol. 2018 Mar 1;314(3):R433-R446. doi: 10.1152/ajpregu.00154.2017. Epub 2017 Nov 22. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 29167165 Free PMC article.
-
Ca2+ responses of pulmonary arterial myocytes to acute hypoxia require release from ryanodine and inositol trisphosphate receptors in sarcoplasmic reticulum.Am J Physiol Lung Cell Mol Physiol. 2012 Jul;303(2):L161-8. doi: 10.1152/ajplung.00348.2011. Epub 2012 May 11. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22582116 Free PMC article.
-
Role of Ryanodine Type 2 Receptors in Elementary Ca2+ Signaling in Arteries and Vascular Adaptive Responses.J Am Heart Assoc. 2019 May 7;8(9):e010090. doi: 10.1161/JAHA.118.010090. J Am Heart Assoc. 2019. PMID: 31030596 Free PMC article.
-
Ca2+ channels, ryanodine receptors and Ca(2+)-activated K+ channels: a functional unit for regulating arterial tone.Acta Physiol Scand. 1998 Dec;164(4):577-87. doi: 10.1046/j.1365-201X.1998.00462.x. Acta Physiol Scand. 1998. PMID: 9887980 Review.
-
Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels.Cell Calcium. 2003 Sep;34(3):211-29. doi: 10.1016/s0143-4160(03)00124-6. Cell Calcium. 2003. PMID: 12887969 Review.
Cited by
-
Gestational Hypoxia and Programing of Lung Metabolism.Front Physiol. 2019 Nov 29;10:1453. doi: 10.3389/fphys.2019.01453. eCollection 2019. Front Physiol. 2019. PMID: 31849704 Free PMC article. Review.
-
Gestational Hypoxia Inhibits Pregnancy-Induced Upregulation of Ca2+ Sparks and Spontaneous Transient Outward Currents in Uterine Arteries Via Heightened Endoplasmic Reticulum/Oxidative Stress.Hypertension. 2020 Sep;76(3):930-942. doi: 10.1161/HYPERTENSIONAHA.120.15235. Epub 2020 Jul 20. Hypertension. 2020. PMID: 32683903 Free PMC article.
-
Long-term hypoxia uncouples Ca2+ and eNOS in bradykinin-mediated pulmonary arterial relaxation.Am J Physiol Regul Integr Comp Physiol. 2018 Jun 1;314(6):R870-R882. doi: 10.1152/ajpregu.00311.2017. Epub 2018 Mar 7. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 29513562 Free PMC article.
-
Long-Term Hypoxia Negatively Influences Ca2+ Signaling in Basilar Arterial Myocytes of Fetal and Adult Sheep.Front Physiol. 2022 Jan 18;12:760176. doi: 10.3389/fphys.2021.760176. eCollection 2021. Front Physiol. 2022. PMID: 35115953 Free PMC article.
-
Gestational long-term hypoxia induces metabolomic reprogramming and phenotypic transformations in fetal sheep pulmonary arteries.Am J Physiol Lung Cell Mol Physiol. 2021 May 1;320(5):L770-L784. doi: 10.1152/ajplung.00469.2020. Epub 2021 Feb 24. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 33624555 Free PMC article.
References
-
- Bonnet S, Hyvelin JM, Bonnet P, Marthan R, Savineau JP. Chronic hypoxia-induced spontaneous and rhythmic contractions in the rat main pulmonary artery. Am J Physiol Lung Cell Mol Physiol 281: L183–L192, 2001 - PubMed
-
- Cheng H, Lederer WJ. Calcium sparks. Physiol Rev 88: 1491–1545, 2008 - PubMed
-
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science 262: 740–744, 1993 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

