Decreases in manganese superoxide dismutase expression and activity contribute to oxidative stress in persistent pulmonary hypertension of the newborn
- PMID: 22962015
- PMCID: PMC3517675
- DOI: 10.1152/ajplung.00098.2012
Decreases in manganese superoxide dismutase expression and activity contribute to oxidative stress in persistent pulmonary hypertension of the newborn
Abstract
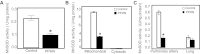
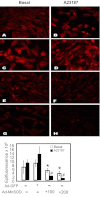
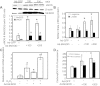
A rapid increase in the synthesis and release of nitric oxide (NO) facilitates the pulmonary vasodilation that occurs during birth-related transition. Alteration of this transition in persistent pulmonary hypertension of the newborn (PPHN) is associated with impaired function of endothelial nitric oxide synthase (eNOS) and an increase in oxidative stress. We investigated the hypothesis that a decrease in expression and activity of mitochondrial localized manganese superoxide dismutase (MnSOD) in pulmonary artery endothelial cells (PAEC) increases oxidative stress and impairs eNOS function in PPHN. We isolated PAEC and pulmonary arteries from fetal lambs with PPHN induced by prenatal ductus arteriosus ligation or sham ligation (control). We investigated MnSOD expression and activity, tyrosine nitration of MnSOD, and mitochondrial O(2)(-) levels in PAEC from control and PPHN lambs. We introduced exogenous MnSOD via an adenoviral vector (ad-MnSOD) transduction into PAEC and pulmonary arteries of PPHN lambs. The effect of ad-MnSOD was investigated on: mitochondrial O(2)(-) levels, MnSOD and eNOS expression and activity, intracellular hydrogen peroxide (H(2)O(2)) levels, and catalase expression in PAEC. MnSOD mRNA and protein levels and activity were decreased and MnSOD tyrosine nitration was increased in PPHN-PAEC. ad-MnSOD transduction of PPHN-PAEC increased its activity two- to threefold, decreased mitochondrial O(2)(-) levels, and increased H(2)O(2) levels and catalase expression. ad-MnSOD transduction improved eNOS expression and function and the relaxation response of PPHN pulmonary arteries. Our observations suggest that decreased MnSOD expression and activity contribute to the endothelial dysfunction observed in PPHN.
Figures




References
-
- Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol Heart Circ Physiol 259: H1921–H1927, 1990 - PubMed
-
- Asayama K, Hayashibe H, Dobashi K, Uchida N, Kobayashi M, Kawaoi A, Kato K. Immunohistochemical study on perinatal development of rat superoxide dismutases in lungs and kidneys. Pediatr Res 29: 487–491, 1991 - PubMed
-
- Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res 92: 683–691, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

