Postnatal exposure history and airways: oxidant stress responses in airway explants
- PMID: 22962062
- PMCID: PMC3547096
- DOI: 10.1165/rcmb.2012-0110OC
Postnatal exposure history and airways: oxidant stress responses in airway explants
Abstract
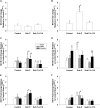
Postnatally, the lung continues to grow and differentiate while interacting with the environment. Exposure to ozone (O(3)) and allergens during postnatal lung development alters structural elements of conducting airways, including innervation and neurokinin abundance. These changes have been linked with development of asthma in a rhesus monkey model. We hypothesized that O(3) exposure resets the ability of the airways to respond to oxidant stress and that this is mediated by changes in the neurokinin-1 receptor (NK-1R). Infant rhesus monkeys received episodic exposure to O(3) biweekly with or without house dust mite antigen (HDMA) from 6 to 12 months of age. Age-matched monkeys were exposed to filtered air (FA). Microdissected airway explants from midlevel airways (intrapulmonary generations 5-8) for four to six animals in each of four groups (FA, O(3), HDMA, and HDMA+O(3)) were tested for NK-1R gene responses to acute oxidant stress using exposure to hydrogen peroxide (1.2 mM), a lipid ozonide (10 μM), or sham treatment for 4 hours in vitro. Airway responses were measured using real-time quantitative RT-PCR of NK-1R and IL-8 gene expression. Basal NK-1R gene expression levels were not different between the exposure groups. Treatment with ozonide or hydrogen peroxide did not change NK-1R gene expression in animals exposed to FA, HDMA, or HDMA+O(3). However, treatment in vitro with lipid ozonide significantly increased NK-1R gene expression in explants from O(3)-exposed animals. We conclude that a history of prior O(3) exposure resets the steady state of the airways to increase the NK-1R response to subsequent acute oxidant stresses.
Figures



Similar articles
-
Ozone-induced airway epithelial cell death, the neurokinin-1 receptor pathway, and the postnatal developing lung.Am J Physiol Lung Cell Mol Physiol. 2014 Sep 15;307(6):L471-81. doi: 10.1152/ajplung.00324.2013. Epub 2014 Jul 25. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 25063800 Free PMC article.
-
Postnatal remodeling of the neural components of the epithelial-mesenchymal trophic unit in the proximal airways of infant rhesus monkeys exposed to ozone and allergen.Toxicol Appl Pharmacol. 2004 Feb 1;194(3):211-20. doi: 10.1016/j.taap.2003.09.025. Toxicol Appl Pharmacol. 2004. PMID: 14761677
-
Repeated episodes of ozone inhalation amplifies the effects of allergen sensitization and inhalation on airway immune and structural development in Rhesus monkeys.Toxicol Appl Pharmacol. 2003 Aug 15;191(1):74-85. doi: 10.1016/s0041-008x(03)00218-7. Toxicol Appl Pharmacol. 2003. PMID: 12915105
-
Organized lymphatic tissue (BALT) in lungs of rhesus monkeys after air pollutant exposure.Anat Rec (Hoboken). 2020 Nov;303(11):2766-2773. doi: 10.1002/ar.24456. Epub 2020 Jul 14. Anat Rec (Hoboken). 2020. PMID: 32445535 Free PMC article. Review.
-
Assessing the health effects and risks associated with children's inhalation exposures--asthma and allergy.J Toxicol Environ Health A. 2008;71(3):196-207. doi: 10.1080/15287390701597897. J Toxicol Environ Health A. 2008. PMID: 18097945 Review.
Cited by
-
Ozone-induced airway epithelial cell death, the neurokinin-1 receptor pathway, and the postnatal developing lung.Am J Physiol Lung Cell Mol Physiol. 2014 Sep 15;307(6):L471-81. doi: 10.1152/ajplung.00324.2013. Epub 2014 Jul 25. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 25063800 Free PMC article.
-
Ozone in the Development of Pediatric Asthma and Atopic Disease.Immunol Allergy Clin North Am. 2022 Nov;42(4):701-713. doi: 10.1016/j.iac.2022.06.001. Epub 2022 Sep 23. Immunol Allergy Clin North Am. 2022. PMID: 36265970 Free PMC article. Review.
-
Key Functions and Therapeutic Prospects of Nur77 in Inflammation Related Lung Diseases.Am J Pathol. 2019 Mar;189(3):482-491. doi: 10.1016/j.ajpath.2018.10.002. Epub 2018 Nov 7. Am J Pathol. 2019. PMID: 30414411 Free PMC article. Review.
-
Nonhuman Primate Models of Respiratory Disease: Past, Present, and Future.ILAR J. 2017 Dec 1;58(2):269-280. doi: 10.1093/ilar/ilx030. ILAR J. 2017. PMID: 29216343 Free PMC article. Review.
-
Ozone-Induced Oxidative Stress, Neutrophilic Airway Inflammation, and Glucocorticoid Resistance in Asthma.Front Immunol. 2021 Feb 26;12:631092. doi: 10.3389/fimmu.2021.631092. eCollection 2021. Front Immunol. 2021. PMID: 33717165 Free PMC article. Review.
References
-
- CDC Asthma prevalence and control characteristics by race/ethnicity: United States (2002). MMWR 2004;53:145–148 - PubMed
-
- Peden DB. The epidemiology and genetics of asthma risk associated with air pollution. J Allergy Clin Immunol 2005;115:213–219 - PubMed
-
- McConnell R, Berhane K, Gilliland F, London SJ, Islam T, Gauderman WJ, Avol E, Margolis HG, Peters JM. Asthma in exercising children exposed to ozone: a cohort study. Lancet 2002;359:386–391 - PubMed
-
- Tager IB, Balmes J, Lurmann F, Ngo L, Alcorn S, Kunzli N. Chronic exposure to ambient ozone and lung function in young adults. Epidemiology 2005;16:751–759 - PubMed
-
- Fanucchi MV, Plopper CG, Evans MJ, Hyde DM, Van Winkle LS, Gershwin LJ, Schelegle ES. Cyclic exposure to ozone alters distal airway development in infant rhesus monkeys. Am J Physiol Lung Cell Mol Physiol 2006;291:L644–L650 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

