Chromosome-specific accumulation of aneuploidy in the aging mouse brain
- PMID: 22962300
- PMCID: PMC3510757
- DOI: 10.1093/hmg/dds375
Chromosome-specific accumulation of aneuploidy in the aging mouse brain
Abstract
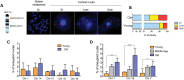
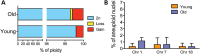
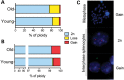
Chromosomal aneuploidy, the gain or loss of whole chromosomes, is a hallmark of pathological conditions and a causal factor of birth defects and cancer. A number of studies indicate that aneuploid cells are present at a high frequency in the brain of mice and humans, suggesting that mosaic aneuploidies are compatible with normal brain function and prompting the question about their consequences. To explore the possible contribution of aneuploidy to functional decline and loss of cognitive functions during aging, we used a quantitative, dual-labeling interphase-fluorescence in situ hybridization approach to compare aneuploidy levels of chromosomes 1, 7, 14, 15, 16, 18, 19 and Y in the cerebral cortex of 4- and 28-month-old mice. We show that aneuploidy accumulates with age in a chromosome-specific manner, with chromosomes 7, 18 and Y most severely affected, i.e. up to 9.8% of non-neuronal brain nuclei in 28-month-old animals for chromosome 18. While at early age, both neuronal and glial cells are affected equally, the age-related increase was limited to the non-neuronal nuclei. No age-related increase in aneuploidy was observed in the cerebellum or in the spleen of the same animals. Extrapolating the average frequencies of aneuploidy from the average over 8 chromosomes to all 20 mouse chromosomes would indicate an almost 50% aneuploidy frequency in aged mouse brain. Such high levels of genome instability could well be a factor in age-related neurodegeneration.
Figures

References
-
- Iourov I.Y., Vorsanova S.G., Yurov Y.B. Chromosomal variation in mammalian neuronal cells: known facts and attractive hypotheses. Int. Rev. Cytol. 2006;249:143–191. doi:10.1016/S0074-7696(06)49003-3. - DOI - PubMed
-
- Suzumori N., Sugiura-Ogasawara M. Genetic factors as a cause of miscarriage. Curr. Med. Chem. 2010;17:3431–3437. - PubMed
-
- Kops G.J., Weaver B.A., Cleveland D.W. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat. Rev. Cancer. 2005;5:773–785. doi:10.1038/nrc1714. - DOI - PubMed
-
- Weaver B.A., Silk A.D., Montagna C., Verdier-Pinard P., Cleveland D.W. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell. 2007;11:25–36. doi:10.1016/j.ccr.2006.12.003. - DOI - PubMed
-
- Jacobs P.A., Court Brown W.M., Doll R. Distribution of human chromosome counts in relation to age. Nature. 1961;191:1178–1180. doi:10.1038/1911178a0. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

