Gβγ inhibits exocytosis via interaction with critical residues on soluble N-ethylmaleimide-sensitive factor attachment protein-25
- PMID: 22962332
- PMCID: PMC3502621
- DOI: 10.1124/mol.112.080507
Gβγ inhibits exocytosis via interaction with critical residues on soluble N-ethylmaleimide-sensitive factor attachment protein-25
Abstract
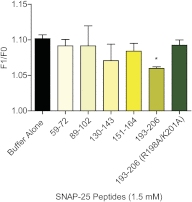
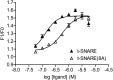
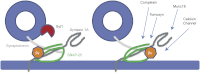
Spatial and temporal regulation of neurotransmitter release is a complex process accomplished by the exocytotic machinery working in tandem with numerous regulatory proteins. G-protein βγ dimers regulate the core process of exocytosis by interacting with the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins soluble N-ethylmaleimide-sensitive factor attachment protein-25 (SNAP-25), syntaxin 1A, and synaptobrevin. Gβγ binding to ternary SNAREs overlaps with calcium-dependent binding of synaptotagmin, inhibiting synaptotagmin-1 binding and fusion of the synaptic vesicle. To further explore the binding sites of Gβγ on SNAP-25, peptides based on the sequence of SNAP-25 were screened for Gβγ binding. Peptides that bound Gβγ were subjected to alanine scanning mutagenesis to determine their relevance to the Gβγ-SNAP-25 interaction. Peptides from this screen were tested in protein-protein interaction assays for their ability to modulate the interaction of Gβγ with SNAP-25. A peptide from the C terminus, residues 193 to 206, significantly inhibited the interaction. In addition, Ala mutants of SNAP-25 residues from the C terminus of SNAP-25, as well as from the amino-terminal region decreased binding to Gβ₁γ₁. When SNAP-25 with eight residues mutated to alanine was assembled with syntaxin 1A, there was significantly reduced affinity of this mutated t-SNARE for Gβγ, but it still interacted with synaptotagmin-1 in a Ca²⁺ -dependent manner and reconstituted evoked exocytosis in botulinum neurotoxin E-treated neurons. However, the mutant SNAP-25 could no longer support 5-hydroxytryptamine-mediated inhibition of exocytosis.
Figures


Similar articles
-
Gβγ directly modulates vesicle fusion by competing with synaptotagmin for binding to neuronal SNARE proteins embedded in membranes.J Biol Chem. 2017 Jul 21;292(29):12165-12177. doi: 10.1074/jbc.M116.773523. Epub 2017 May 17. J Biol Chem. 2017. PMID: 28515322 Free PMC article.
-
Gbetagamma interferes with Ca2+-dependent binding of synaptotagmin to the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex.Mol Pharmacol. 2007 Nov;72(5):1210-9. doi: 10.1124/mol.107.039446. Epub 2007 Aug 22. Mol Pharmacol. 2007. PMID: 17715396
-
Gbetagamma acts at the C terminus of SNAP-25 to mediate presynaptic inhibition.Nat Neurosci. 2005 May;8(5):597-605. doi: 10.1038/nn1439. Epub 2005 Apr 17. Nat Neurosci. 2005. PMID: 15834421
-
Rab3 and synaptotagmin proteins in the regulation of vesicle fusion and neurotransmitter release.Life Sci. 2022 Nov 15;309:120995. doi: 10.1016/j.lfs.2022.120995. Epub 2022 Sep 24. Life Sci. 2022. PMID: 36167148 Review.
-
GPCR regulation of secretion.Pharmacol Ther. 2018 Dec;192:124-140. doi: 10.1016/j.pharmthera.2018.07.005. Epub 2018 Jul 26. Pharmacol Ther. 2018. PMID: 30056056 Free PMC article. Review.
Cited by
-
SNAP25 differentially contributes to Gi/o-coupled receptor function at glutamatergic synapses in the nucleus accumbens.Front Cell Neurosci. 2023 May 2;17:1165261. doi: 10.3389/fncel.2023.1165261. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37206665 Free PMC article.
-
Quantitative Multiple-Reaction Monitoring Proteomic Analysis of Gβ and Gγ Subunits in C57Bl6/J Brain Synaptosomes.Biochemistry. 2017 Oct 10;56(40):5405-5416. doi: 10.1021/acs.biochem.7b00433. Epub 2017 Sep 21. Biochemistry. 2017. PMID: 28880079 Free PMC article.
-
Gβγ Binds to the Extreme C Terminus of SNAP25 to Mediate the Action of Gi/o-Coupled G Protein-Coupled Receptors.Mol Pharmacol. 2016 Jan;89(1):75-83. doi: 10.1124/mol.115.101600. Epub 2015 Oct 30. Mol Pharmacol. 2016. PMID: 26519224 Free PMC article.
-
Gβγ directly modulates vesicle fusion by competing with synaptotagmin for binding to neuronal SNARE proteins embedded in membranes.J Biol Chem. 2017 Jul 21;292(29):12165-12177. doi: 10.1074/jbc.M116.773523. Epub 2017 May 17. J Biol Chem. 2017. PMID: 28515322 Free PMC article.
-
The in vivo specificity of synaptic Gβ and Gγ subunits to the α2a adrenergic receptor at CNS synapses.Sci Rep. 2019 Feb 8;9(1):1718. doi: 10.1038/s41598-018-37222-1. Sci Rep. 2019. PMID: 30737458 Free PMC article.
References
-
- Binz T, Blasi J, Yamasaki S, Baumeister A, Link E, Südhof TC, Jahn R, Niemann H. (1994) Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J Biol Chem 269:1617–1620 - PubMed
-
- Blackmer T, Larsen EC, Bartleson C, Kowalchyk JA, Yoon EJ, Preininger AM, Alford S, Hamm HE, Martin TF. (2005) G protein βγ directly regulates SNARE protein fusion machinery for secretory granule exocytosis. Nat Neurosci 8:421–425 - PubMed
-
- Blackmer T, Larsen EC, Takahashi M, Martin TF, Alford S, Hamm HE. (2001) G protein βγ subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science 292:293–297 - PubMed
-
- Brose N, Petrenko AG, Südhof TC, Jahn R. (1992) Synaptotagmin—a calcium sensor on the synaptic vesicle surface. Science 256(5059):1021–1025 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

