Lessons Learned while Traversing the Welwitindolinone Alkaloids Obstacle Course
- PMID: 22962500
- PMCID: PMC3434954
- DOI: 10.1016/j.tet.2011.09.088
Lessons Learned while Traversing the Welwitindolinone Alkaloids Obstacle Course
Abstract
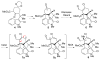
We recount several unexpected results observed in the course of our work toward the synthesis of welwitindolinone alkaloids. The surprising results provide an opportunity to refine one's understanding of the interplay between chemical structure and reactivity.
Figures
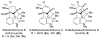
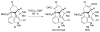
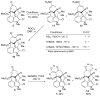
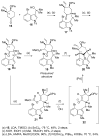
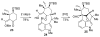
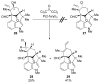
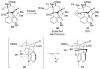
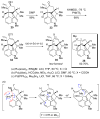
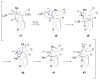
References
-
- Stratmann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GMLS, Shaffer Smith CD, Smitka TA. J Am Chem Soc. 1994;116:9935–9942.
- Jimenez JI, Huber U, Moore RE, Patterson GML. J Nat Prod. 1999;62:569–572. - PubMed
-
-
For approaches toward the welwitindolinones (1–3), see: Konopelski JP, Deng H, Schiemann K, Keane JM, Olmstead MM. Synlett. 1998:1105–1107.Wood JL, Holubec AA, Stoltz BM, Weiss MM, Dixon JA, Doan BD, Shamji MF, Chen JM, Heffron TP. J Am Chem Soc. 1999;121:6326–6327.Kaoudi T, Ouiclet-Sire B, Seguin S, Zard SZ. Angew Chem, Int Ed. 2000;39:731–733.Deng H, Konopelski JP. Org Lett. 2001;3:3001–3004.Jung ME, Slowinski F. Tetrahedron Lett. 2001;42:6835–6838.López-Alvarado P, García-Granda S, Àlvarez-Rúa C, Avendaño C. Eur J Org Chem. 2002:1702–1707.Ready JM, Reisman SE, Hirata M, Weiss MM, Tamaki K, Ovaska TV, Wood JL. Angew Chem, Int Ed. 2004;43:1270–1272.Baudoux J, Blake AJ, Simpkins NS. Org Lett. 2005;7:4087–4089.Greshock TJ, Funk RL. Org Lett. 2006;8:2643–2645.Lauchli R, Shea KJ. Org Lett. 2006;8:5287–5289.Xia J, Brown LE, Konopelski JP. J Org Chem. 2007;72:6885–6890.Richter JM, Ishihara Y, Masuda T, Whitefield BW, Llamas T, Pohjakallio A, Baran PS. J Am Chem Soc. 2008;130:17938–17954.Boissel V, Simpkins NS, Bhalay G, Blake AJ, Lewis W. Chem Commun. 2009:1398–1400.Boissel V, Simpkins NS, Bhalay G. Tetrahedron Lett. 2009;50:3283–3286.Tian X, Huters AD, Douglas CJ, Garg NK. Org Lett. 2009;11:2349–2351.Trost BM, McDougall PJ. Org Lett. 2009;11:3782–3785.Brailsford JA, Lauchli R, Shea KJ. Org Lett. 2009;11:5330–5333.Freeman DB, et al. Tetrahedron. 2010;66:6647–6655.Heidebrecht RW, Jr, Gulledge B, Martin SF. Org Lett. 2010;12:2492–2495.Ruiz M, López-Alvarado P, Menéndez JC. Org Biomol Chem. 2010;8:4521–4523.
-
-
-
Total syntheses of welwitindolinone A: Baran PS, Richter JM. J Am Chem Soc. 2005;127:15394–15396.Reisman SE, Ready JM, Hasuoka A, Smith CJ, Wood JL. J Am Chem Soc. 2006;128:1448–1449.For the synthesis of welwitindolinone D (3), see ref . (d) While the present manuscript was under review for publication, a report appeared in J. Am. Chem. Soc., ASAP on the total synthesis of welwitindolinone C (2): Huters AD, Quasdorf KW, Styduhar ED, Garg NK. J Am Chem Soc. 2011 doi: 10.1021/ja206538k.
-
-
-
For a review on the Vilsmeier–Haack reaction, see: Meth-Con O, Stanforth SP. Comp Org Syn. 1991;2:777–794.For an example of deconjugative methylation of a cyclic enal, see: Burnell RH, Côté C, Théberge N. J Nat Prod. 1993;56:1459–1467.
-
Grants and funding
LinkOut - more resources
Full Text Sources
