Immobilized Carbonic Anhydrase on Hollow Fiber Membranes Accelerates CO(2) Removal from Blood
- PMID: 22962517
- PMCID: PMC3433239
- DOI: 10.1016/j.memsci.2012.02.006
Immobilized Carbonic Anhydrase on Hollow Fiber Membranes Accelerates CO(2) Removal from Blood
Abstract
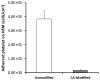
Current artificial lungs and respiratory assist devices designed for carbon dioxide removal (CO(2)R) are limited in their efficiency due to the relatively small partial pressure difference across gas exchange membranes. To offset this underlying diffusional challenge, bioactive hollow fiber membranes (HFMs) increase the carbon dioxide diffusional gradient through the immobilized enzyme carbonic anhydrase (CA), which converts bicarbonate to CO(2) directly at the HFM surface. In this study, we tested the impact of CA-immobilization on HFM CO(2) removal efficiency and thromboresistance in blood. Fiber surface modification with radio frequency glow discharge (RFGD) introduced hydroxyl groups, which were activated by 1M CNBr while 1.5M TEA was added drop wise over the activation time course, then incubation with a CA solution covalently linked the enzyme to the surface. The bioactive HFMs were then potted in a model gas exchange device (0.0084 m(2)) and tested in a recirculation loop with a CO(2) inlet of 50mmHg under steady blood flow. Using an esterase activity assay, CNBr chemistry with TEA resulted in 0.99U of enzyme activity, a 3.3 fold increase in immobilized CA activity compared to our previous method. These bioactive HFMs demonstrated 108 ml/min/m(2) CO(2) removal rate, marking a 36% increase compared to unmodified HFMs (p < 0.001). Thromboresistance of CA-modified HFMs was assessed in terms of adherent platelets on surfaces by using lactate dehydrogenase (LDH) assay as well as scanning electron microscopy (SEM) analysis. Results indicated HFMs with CA modification had 95% less platelet deposition compared to unmodified HFM (p < 0.01). Overall these findings revealed increased CO(2) removal can be realized through bioactive HFMs, enabling a next generation of more efficient CO(2) removal intravascular and paracorporeal respiratory assist devices.
Figures





References
-
- Tremblay LN, Slutsky AS. Applied Physiology in Intensive Care Medicine. Berlin, Heidelberg: Springer Berlin Heidelberg; 2006. Ventilator-induced lung injury: from the bench to the bedside; pp. 357–66. - PubMed
-
- Ware LB, Matthay MA. The Acute Respiratory Distress Syndrome. N Engl J Med. 2000;342(18):1334–49. - PubMed
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and Outcomes of Acute Lung Injury. N Engl J Med. 2005;353(16):1685–93. - PubMed
-
- Brower R, Matthay MA. Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. New England Journal of Medicine. 2000 May 4;342(18):1301–8. - PubMed
-
- Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, et al. Tidal Hyperinflation During Low Tidal Volume Ventilation in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2006 Oct 12;:200607-915OC. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
