The effect of irradiation wavelengths and the crystal structures of titanium dioxide on the formation of singlet oxygen for bacterial killing
- PMID: 22962531
- PMCID: PMC3432823
- DOI: 10.3164/jcbn.11-22.
The effect of irradiation wavelengths and the crystal structures of titanium dioxide on the formation of singlet oxygen for bacterial killing
Abstract
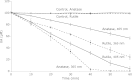
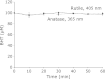
Safe and effective methods for oral bacterial disinfection have been desired, since bacteria cause many infectious diseases such as dental caries, periodontal disease, and endodontic infections. Singlet oxygen ((1)O(2)) is attractive, because it is toxic to prokaryotic cells, but not to eukaryotic cells. We selected irradiation of titanium dioxide (TiO(2)) as a source of (1)O(2), because it has been used in sunscreens and cosmetic products without complications. In order to establish the optimal oral photodynamic therapy conditions, we measured the rate of (1)O(2) formation from the irradiated anatase or rutile forms of TiO(2) using 365 or 405 nm lamps. The rate of (1)O(2) formation decreased in the following order: anatase, 365 nm > rutile, 405 nm > rutile, 365 nm > anatase, 405 nm. Therefore, we concluded that irradiation of the rutile form of TiO(2) by a 405 nm lamp is the most favorable photodynamic therapy condition, because visible light is more desirable than UV light from the viewpoint of patient safety. We also confirmed that there was no direct HO(•) formation from the irradiated TiO(2).
Keywords: 405 nm; electron spin resonance spectroscopy; photodynamic therapy; singlet oxygen; titanium dioxide.
Figures



Similar articles
-
The different behavior of rutile and anatase nanoparticles in forming oxy radicals upon illumination with visible light: an EPR study.Photochem Photobiol. 2012 Jan-Feb;88(1):14-20. doi: 10.1111/j.1751-1097.2011.01015.x. Epub 2011 Nov 3. Photochem Photobiol. 2012. PMID: 21988075
-
The crystal structure of titanium dioxide nanoparticles influences immune activity in vitro and in vivo.Part Fibre Toxicol. 2018 Jan 30;15(1):9. doi: 10.1186/s12989-018-0245-5. Part Fibre Toxicol. 2018. PMID: 29382351 Free PMC article.
-
Titanium Dioxide Particle Type and Concentration Influence the Inflammatory Response in Caco-2 Cells.Int J Mol Sci. 2016 Apr 16;17(4):576. doi: 10.3390/ijms17040576. Int J Mol Sci. 2016. PMID: 27092499 Free PMC article.
-
Bactericidal effects and mechanisms of visible light-responsive titanium dioxide photocatalysts on pathogenic bacteria.Arch Immunol Ther Exp (Warsz). 2012 Aug;60(4):267-75. doi: 10.1007/s00005-012-0178-x. Epub 2012 Jun 8. Arch Immunol Ther Exp (Warsz). 2012. PMID: 22678625 Review.
-
Ferrate(VI) enhanced photocatalytic oxidation of pollutants in aqueous TiO2 suspensions.Environ Sci Pollut Res Int. 2010 Feb;17(2):453-61. doi: 10.1007/s11356-009-0170-0. Epub 2009 Jun 3. Environ Sci Pollut Res Int. 2010. PMID: 19495821 Review.
Cited by
-
Current trends and research advances on the application of TiO2 nanoparticles in dentistry: How far are we from clinical translation?Heliyon. 2025 Jan 22;11(3):e42169. doi: 10.1016/j.heliyon.2025.e42169. eCollection 2025 Feb 15. Heliyon. 2025. PMID: 39991247 Free PMC article. Review.
-
On the Discoloration of Methylene Blue by Visible Light.J Fluoresc. 2019 Jan;29(1):15-25. doi: 10.1007/s10895-018-2304-6. Epub 2018 Oct 26. J Fluoresc. 2019. PMID: 30361861
References
-
- Wilson M. Susceptibility of oral bacteria biofilm to antimicrobal agents. J Med Microbiol. 1996;44:79–87. - PubMed
-
- Soukos NS, Goodson JM. Photodynamic therapy in the control of oral biofilms. Periodontol. 2000;55:143–166. - PubMed
-
- Nery EB, Meister F, Jr., Ellinger RF, Eslami A, McNa mara TJ. Prevalance of medical problems in periodontal patients obtained from three different populations. J Periodontol. 1987;58:564–568. - PubMed
-
- Terpennig MS, Taylor GW, Lopatin DE, Kerr C, Dominguez BL, Loesche WJ. Aspiration pneumonia: dental and oral risk factors in an older veteran population. J Am Geriatr Soc. 2001;49:557–563. - PubMed
-
- Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontal. 2001;6:99–112. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

