PPAR-γ regulates carnitine homeostasis and mitochondrial function in a lamb model of increased pulmonary blood flow
- PMID: 22962578
- PMCID: PMC3433474
- DOI: 10.1371/journal.pone.0041555
PPAR-γ regulates carnitine homeostasis and mitochondrial function in a lamb model of increased pulmonary blood flow
Abstract
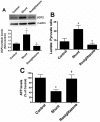
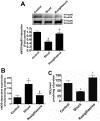
Objective: Carnitine homeostasis is disrupted in lambs with endothelial dysfunction secondary to increased pulmonary blood flow (Shunt). Our recent studies have also indicated that the disruption in carnitine homeostasis correlates with a decrease in PPAR-γ expression in Shunt lambs. Thus, this study was carried out to determine if there is a causal link between loss of PPAR-γ signaling and carnitine dysfunction, and whether the PPAR-γ agonist, rosiglitazone preserves carnitine homeostasis in Shunt lambs.
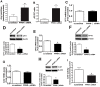
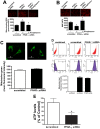
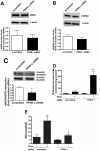
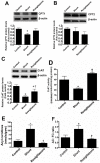
Methods and results: siRNA-mediated PPAR-γ knockdown significantly reduced carnitine palmitoyltransferases 1 and 2 (CPT1 and 2) and carnitine acetyltransferase (CrAT) protein levels. This decrease in carnitine regulatory proteins resulted in a disruption in carnitine homeostasis and induced mitochondrial dysfunction, as determined by a reduction in cellular ATP levels. In turn, the decrease in cellular ATP attenuated NO signaling through a reduction in eNOS/Hsp90 interactions and enhanced eNOS uncoupling. In vivo, rosiglitazone treatment preserved carnitine homeostasis and attenuated the development of mitochondrial dysfunction in Shunt lambs maintaining ATP levels. This in turn preserved eNOS/Hsp90 interactions and NO signaling.
Conclusion: Our study indicates that PPAR-γ signaling plays an important role in maintaining mitochondrial function through the regulation of carnitine homeostasis both in vitro and in vivo. Further, it identifies a new mechanism by which PPAR-γ regulates NO signaling through Hsp90. Thus, PPAR-γ agonists may have therapeutic potential in preventing the endothelial dysfunction in children with increased pulmonary blood flow.
Conflict of interest statement
Figures

Similar articles
-
Altered carnitine homeostasis is associated with decreased mitochondrial function and altered nitric oxide signaling in lambs with pulmonary hypertension.Am J Physiol Lung Cell Mol Physiol. 2008 Jan;294(1):L46-56. doi: 10.1152/ajplung.00247.2007. Epub 2007 Nov 16. Am J Physiol Lung Cell Mol Physiol. 2008. PMID: 18024721 Free PMC article.
-
Disruption of endothelial cell mitochondrial bioenergetics in lambs with increased pulmonary blood flow.Antioxid Redox Signal. 2013 May 10;18(14):1739-52. doi: 10.1089/ars.2012.4806. Epub 2013 Mar 14. Antioxid Redox Signal. 2013. PMID: 23244702 Free PMC article.
-
Role of carnitine acetyl transferase in regulation of nitric oxide signaling in pulmonary arterial endothelial cells.Int J Mol Sci. 2012 Dec 21;14(1):255-72. doi: 10.3390/ijms14010255. Int J Mol Sci. 2012. PMID: 23344032 Free PMC article.
-
L-carnitine preserves endothelial function in a lamb model of increased pulmonary blood flow.Pediatr Res. 2013 Jul;74(1):39-47. doi: 10.1038/pr.2013.71. Epub 2013 Apr 29. Pediatr Res. 2013. PMID: 23628882 Free PMC article.
-
Carnitine O-Acetyltransferase as a Central Player in Lipid and Branched-Chain Amino Acid Metabolism, Epigenetics, Cell Plasticity, and Organelle Function.Biomolecules. 2025 Feb 2;15(2):216. doi: 10.3390/biom15020216. Biomolecules. 2025. PMID: 40001519 Free PMC article. Review.
Cited by
-
Nutrigenomics and Beef Quality: A Review about Lipogenesis.Int J Mol Sci. 2016 Jun 10;17(6):918. doi: 10.3390/ijms17060918. Int J Mol Sci. 2016. PMID: 27294923 Free PMC article. Review.
-
PGC1α-Mediated Metabolic Reprogramming Drives the Stemness of Pancreatic Precursor Lesions.Clin Cancer Res. 2021 Oct 1;27(19):5415-5429. doi: 10.1158/1078-0432.CCR-20-5020. Clin Cancer Res. 2021. PMID: 34172498 Free PMC article.
-
Altered Carnitine Homeostasis in Children With Increased Pulmonary Blood Flow Due to Ventricular Septal Defects.Pediatr Crit Care Med. 2017 Oct;18(10):931-934. doi: 10.1097/PCC.0000000000001275. Pediatr Crit Care Med. 2017. PMID: 28723882 Free PMC article.
-
Acyl-CoA thioesterase 13 (ACOT13) attenuates the progression of autosomal dominant polycystic kidney disease in vitro via triggering mitochondrial-related cell apoptosis.Aging (Albany NY). 2024 Aug 21;16(16):11877-11892. doi: 10.18632/aging.206054. Epub 2024 Aug 21. Aging (Albany NY). 2024. PMID: 39172111 Free PMC article.
-
Prepartum dietary energy intake alters adipose tissue transcriptome profiles during the periparturient period in Holstein dairy cows.J Anim Sci Biotechnol. 2020 Jan 3;11:1. doi: 10.1186/s40104-019-0409-7. eCollection 2020. J Anim Sci Biotechnol. 2020. PMID: 31908775 Free PMC article.
References
-
- Sharma S, Grobe AC, Wiseman DA, Kumar S, Englaish M, et al. (2007) Lung antioxidant enzymes are regulated by development and increased pulmonary blood flow. Am J Physiol Lung Cell Mol Physiol 293: L960–971. - PubMed
-
- Kersten S, Desvergne B, Wahli W (2000) Roles of PPARs in health and disease. Nature 405: 421–424. - PubMed
-
- Vamecq J, Latruffe N (1999) Medical significance of peroxisome proliferator-activated receptors. Lancet 354: 141–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 HD057406/HD/NICHD NIH HHS/United States
- R01 HL070061/HL/NHLBI NIH HHS/United States
- R01 HL061284/HL/NHLBI NIH HHS/United States
- R21HD057406/HD/NICHD NIH HHS/United States
- HL67841/HL/NHLBI NIH HHS/United States
- HL72123/HL/NHLBI NIH HHS/United States
- R01 HL072123/HL/NHLBI NIH HHS/United States
- R01 HL067841/HL/NHLBI NIH HHS/United States
- 5T32-HL-06699/HL/NHLBI NIH HHS/United States
- HL61284/HL/NHLBI NIH HHS/United States
- HL70061/HL/NHLBI NIH HHS/United States
- HL60190/HL/NHLBI NIH HHS/United States
- 11SDG7460024/PHS HHS/United States
- R01 HL060190/HL/NHLBI NIH HHS/United States
- T32 HD049303/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

