Expression of TRPV1 channels after nerve injury provides an essential delivery tool for neuropathic pain attenuation
- PMID: 22962595
- PMCID: PMC3433461
- DOI: 10.1371/journal.pone.0044023
Expression of TRPV1 channels after nerve injury provides an essential delivery tool for neuropathic pain attenuation
Abstract
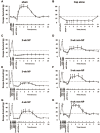
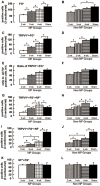
Increased expression of the transient receptor potential vanilloid 1 (TRPV1) channels, following nerve injury, may facilitate the entry of QX-314 into nociceptive neurons in order to achieve effective and selective pain relief. In this study we hypothesized that the level of QX-314/capsaicin (QX-CAP)--induced blockade of nocifensive behavior could be used as an indirect in-vivo measurement of functional expression of TRPV1 channels. We used the QX-CAP combination to monitor the functional expression of TRPV1 in regenerated neurons after inferior alveolar nerve (IAN) transection in rats. We evaluated the effect of this combination on pain threshold at different time points after IAN transection by analyzing the escape thresholds to mechanical stimulation of lateral mental skin. At 2 weeks after IAN transection, there was no QX-CAP mediated block of mechanical hyperalgesia, implying that there was no functional expression of TRPV1 channels. These results were confirmed immunohistochemically by staining of regenerated trigeminal ganglion (TG) neurons. This suggests that TRPV1 channel expression is an essential necessity for the QX-CAP mediated blockade. Furthermore, we show that 3 and 4 weeks after IAN transection, application of QX-CAP produced a gradual increase in escape threshold, which paralleled the increased levels of TRPV1 channels that were detected in regenerated TG neurons. Immunohistochemical analysis also revealed that non-myelinated neurons regenerated slowly compared to myelinated neurons following IAN transection. We also show that TRPV1 expression shifted towards myelinated neurons. Our findings suggest that nerve injury modulates the TRPV1 expression pattern in regenerated neurons and that the effectiveness of QX-CAP induced blockade depends on the availability of functional TRPV1 receptors in regenerated neurons. The results of this study also suggest that the QX-CAP based approach can be used as a new behavioral tool to detect dynamic changes in TRPV1 expression, in various pathological conditions.
Conflict of interest statement
Figures


Similar articles
-
A novel approach for detection of functional expression of TRPV1 channels on regenerated neurons following nerve injury.J Oral Sci. 2020 Mar 28;62(2):136-139. doi: 10.2334/josnusd.19-0356. Epub 2020 Mar 19. J Oral Sci. 2020. PMID: 32074545
-
Selectively targeting pain in the trigeminal system.Pain. 2010 Jul;150(1):29-40. doi: 10.1016/j.pain.2010.02.016. Epub 2010 Mar 16. Pain. 2010. PMID: 20236764 Free PMC article.
-
Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers.Nature. 2007 Oct 4;449(7162):607-10. doi: 10.1038/nature06191. Nature. 2007. PMID: 17914397
-
Differential effects of TRPV channel block on polymodal activation of rat cutaneous nociceptors in vitro.Exp Brain Res. 2009 Jun;196(1):31-44. doi: 10.1007/s00221-009-1808-3. Epub 2009 Apr 30. Exp Brain Res. 2009. PMID: 19404626 Review.
-
Targeting the TRPV1 pain pathway in osteoarthritis of the knee.Expert Opin Ther Targets. 2024 Oct;28(10):843-856. doi: 10.1080/14728222.2024.2416961. Epub 2024 Oct 25. Expert Opin Ther Targets. 2024. PMID: 39450875 Review.
Cited by
-
Targeting Peripherally Restricted Cannabinoid Receptor 1, Cannabinoid Receptor 2, and Endocannabinoid-Degrading Enzymes for the Treatment of Neuropathic Pain Including Neuropathic Orofacial Pain.Int J Mol Sci. 2020 Feb 20;21(4):1423. doi: 10.3390/ijms21041423. Int J Mol Sci. 2020. PMID: 32093166 Free PMC article. Review.
-
Resiniferatoxin (RTX) causes a uniquely protracted musculoskeletal hyperalgesia in mice by activation of TRPV1 receptors.J Pain. 2013 Dec;14(12):1629-41. doi: 10.1016/j.jpain.2013.07.021. Epub 2013 Nov 1. J Pain. 2013. PMID: 24188863 Free PMC article.
-
Boric Acid Diminishes Sciatic Nerve Injury-Induced Apoptosis, Oxidative Stress, and Pain via The Block of TRPV1 Channel in Mice.Biol Trace Elem Res. 2025 Jun 28. doi: 10.1007/s12011-025-04698-8. Online ahead of print. Biol Trace Elem Res. 2025. PMID: 40580239
-
The Role of Transient Receptor Potential (TRP) Channels in the Transduction of Dental Pain.Int J Mol Sci. 2019 Jan 27;20(3):526. doi: 10.3390/ijms20030526. Int J Mol Sci. 2019. PMID: 30691193 Free PMC article. Review.
-
Primary sensory neuron-specific interference of TRPV1 signaling by AAV-encoded TRPV1 peptide aptamer attenuates neuropathic pain.Mol Pain. 2017 Jan-Dec;13:1744806917717040. doi: 10.1177/1744806917717040. Mol Pain. 2017. PMID: 28604222 Free PMC article.
References
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, et al. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824. - PubMed
-
- Tominaga M, Caterina MJ (2004) Thermosensation and pain. J Neurobiol 61: 3–12. - PubMed
-
- Tominaga M, Tominaga T (2005) Structure and function of TRPV1. Pflugers Arch 451: 143–150. - PubMed
-
- Biggs JE, Yates JM, Loescher AR, Clayton NM, Boissonade FM, et al. (2007) Changes in vanilloid receptor 1 (TRPV1) expression following lingual nerve injury. Eur J Pain 11: 192–201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

