Toll-like receptor 4 and high-mobility group box 1 are critical mediators of tissue injury and survival in a mouse model for heatstroke
- PMID: 22962600
- PMCID: PMC3433483
- DOI: 10.1371/journal.pone.0044100
Toll-like receptor 4 and high-mobility group box 1 are critical mediators of tissue injury and survival in a mouse model for heatstroke
Abstract
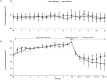
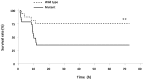
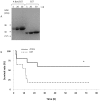
The molecular mechanisms that initiate the inflammatory response in heatstroke and their relation with tissue injury and lethality are not fully elucidated. We examined whether endogenous ligands released by damaged/stressed cells such as high-mobility group box 1 (HMGB1) signaling through Toll-like receptor 4 (TLR4) may play a pathogenic role in heatstroke. Mutant TLR4-defective (C3H/HeJ) and wild type (C3H/HeOuJ) mice were subjected to heat stress in an environmental chamber pre-warmed at 43.5 °C until their core temperature reached 42.7°C, which was taken as the onset of heatstroke. The animals were then allowed to recover passively at ambient temperature. A sham-heated group served as a control. Mutant mice displayed more histological liver damage and higher mortality compared with wild type mice (73% vs. 27%, respectively, P<0.001). Compared to wild type mice, mutant mice exhibited earlier plasma release of markers of systemic inflammation such as HMGB1 (206 ± 105 vs. 63 ± 21 ng/ml; P = 0.0018 and 209 ± 100 vs. 46 ± 32 ng/ml; P<0.0001), IL-6 (144 ± 40 vs. 46 ± 20 pg/ml; P<0.001 and 184 ± 21 vs. 84 ± 54 pg/ml; P = 0.04), and IL-1β (27 ± 4 vs. 1.7 ± 2.3 pg/ml; P<0.0001 at 1 hour). Both strains of mice displayed early release of HMGB1 into the circulation upstream of IL-1β and IL-6 responses which remained elevated up to 24 h. Specific inhibition of HMGB1 activity with DNA-binding A Box (600 µg/mouse) protected the mutant mice against the lethal effect of heat stress (60% A Box vs. 18% GST protein, P = 0.04). These findings suggest a protective role for the TLR4 in the host response to severe heat stress. They also suggest that HMGB1 is an early mediator of inflammation, tissue injury and lethality in heatstroke in the presence of defective TLR4 signaling.
Conflict of interest statement
Figures


References
-
- Robine JM, Cheung SL, Le Roy S, Van Oyen H, Griffiths C, et al. (2008) Death toll exceeded 70,000 in Europe during the summer of 2003. C R Biol 331: 171–178. - PubMed
-
- Semenza JC, Rubin CH, Falter KH, Selanikio JD, Flanders WD, et al. (1996) Heat-related deaths during the July 1995 heat wave in Chicago. N Engl J Med 335: 84–90. - PubMed
-
- Bouchama A, Knochel JP (2002) Heat stroke. N Engl J Med 346: 1978–1988. - PubMed
-
- Roberts GT, Ghebeh H, Chishti MA, Al-Mohanna F, El-Sayed R, et al. (2008) Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heatstroke: a study in baboon model. Arterioscler Thromb Vasc Biol 28: 1130–1136. - PubMed
-
- Bouchama A, Ollivier V, Roberts G, Al Mohanna F, de Prost D, et al. (2005) Experimental heatstroke in baboon: analysis of the systemic inflammatory response. Shock 24: 332–335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

