CD86 and IL-12p70 are key players for T helper 1 polarization and natural killer cell activation by Toll-like receptor-induced dendritic cells
- PMID: 22962607
- PMCID: PMC3433478
- DOI: 10.1371/journal.pone.0044266
CD86 and IL-12p70 are key players for T helper 1 polarization and natural killer cell activation by Toll-like receptor-induced dendritic cells
Abstract
Background: Dendritic cells (DCs) determine the activation and polarization of T cells via expression of costimulatory molecules and secretion of cytokines. The function of DCs derived from monocytes ex vivo strongly depends on the composition of the maturation cocktail used.
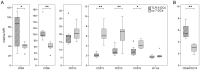
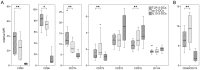
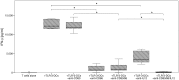
Methodology/principal findings: We analyzed the effect of costimulatory molecule expression and cytokine secretion by DCs on T and natural killer (NK) cell activation by conducting a head-to-head comparison of a Toll-like receptor (TLR) agonist-based cocktail with the standard combination of proinflammatory cytokines or IL-10 alone. We could show that TLR-induced DCs are characterized by a predominance of costimulatory over coinhibitory molecules and by high secretion of IL-12p70, but not IL-10. Functionally, these signals translated into an increase in IFN-γ secreting Th1 cells and a decrease in regulatory T cells. T cell activation and polarization were dependent on IL-12p70 and CD86, but remarkably not on CD80 signaling. By means of IL-12p70 secretion, only TLR-induced DCs activated NK cells.
Conclusions/significance: TLR-matured DCs are highly suitable for application in immunotherapeutic strategies that rely on strong type 1 polarization and NK cell activation. Their effects particularly depend on high CD86 expression and IL-12p70 secretion.
Conflict of interest statement
Figures



References
-
- Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392: 245–252. - PubMed
-
- Schuler G, Schuler-Thurner B, Steinman RM (2003) The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol 15: 138–147. - PubMed
-
- Banchereau J, Palucka AK (2005) Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol 5: 296–306. - PubMed
-
- Melief CJ (2008) Cancer immunotherapy by dendritic cells. Immunity 29: 372–383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

