Lipid Nanoparticles Containing siRNA Synthesized by Microfluidic Mixing Exhibit an Electron-Dense Nanostructured Core
- PMID: 22962627
- PMCID: PMC3434764
- DOI: 10.1021/jp303267y
Lipid Nanoparticles Containing siRNA Synthesized by Microfluidic Mixing Exhibit an Electron-Dense Nanostructured Core
Abstract
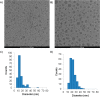
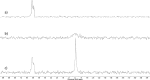
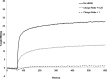
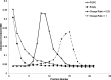
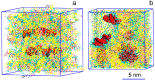
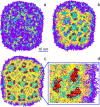
Lipid nanoparticles (LNP) containing ionizable cationic lipids are the leading systems for enabling therapeutic applications of siRNA; however, the structure of these systems has not been defined. Here we examine the structure of LNP siRNA systems containing DLinKC2-DMA(an ionizable cationic lipid), phospholipid, cholesterol and a polyethylene glycol (PEG) lipid formed using a rapid microfluidic mixing process. Techniques employed include cryo-transmission electron microscopy, (31)P NMR, membrane fusion assays, density measurements, and molecular modeling. The experimental results indicate that these LNP siRNA systems have an interior lipid core containing siRNA duplexes complexed to cationic lipid and that the interior core also contains phospholipid and cholesterol. Consistent with experimental observations, molecular modeling calculations indicate that the interior of LNP siRNA systems exhibits a periodic structure of aqueous compartments, where some compartments contain siRNA. It is concluded that LNP siRNA systems formulated by rapid mixing of an ethanol solution of lipid with an aqueous medium containing siRNA exhibit a nanostructured core. The results give insight into the mechanism whereby LNP siRNA systems are formed, providing an understanding of the high encapsulation efficiencies that can be achieved and information on methods of constructing more sophisticated LNP systems.
Figures



References
-
- Zimmermann T. S.; Lee A. C. H.; Akinc A.; Bramlage B.; Bumcrot D.; Fedoruk M. N.; Harborth J.; Heyes J. A.; Jeffs L. B.; John M.; et al. Nature 2006, 441, 111. - PubMed
-
- Semple S. C.; Akinc A.; Chen J.; Sandhu A. P.; Mui B. L.; Cho C. K.; Sah D. W. Y.; Stebbing D.; Crosley E. J.; Yaworski E.; et al. Nat. Biotechnol. 2010, 28, 172. - PubMed
-
- Jeffs L. B.; Palmer L. R.; Ambegia E. G.; Giesbrecht C.; Ewanick S.; MacLachlan I. Pharm. Res. 2005, 22, 362. - PubMed
-
- Crawford R.; Dogdas B.; Keough E.; Haas R. M.; Wepukhulu W.; Krotzer S.; Burke P. a; Sepp-Lorenzino L.; Bagchi A.; Howell B. J. Int. J. Pharm. 2011, 403, 237. - PubMed
-
- Zhigaltsev I. V.; Maurer N.; Edwards K.; Karlsson G.; Cullis P. R. J. Controlled Release 2006, 110, 378. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
