Mechanism of phytohormone involvement in feedback regulation of cotton leaf senescence induced by potassium deficiency
- PMID: 22962680
- PMCID: PMC3467299
- DOI: 10.1093/jxb/ers238
Mechanism of phytohormone involvement in feedback regulation of cotton leaf senescence induced by potassium deficiency
Abstract
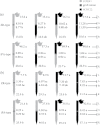
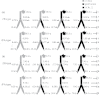
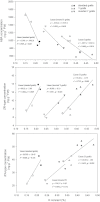
To elucidate the phytohormonal basis of the feedback regulation of leaf senescence induced by potassium (K) deficiency in cotton (Gossypium hirsutum L.), two cultivars contrasting in sensitivity to K deficiency were self- and reciprocally grafted hypocotyl-to-hypocotyl, using standard grafting (one scion grafted onto one rootstock), Y grafting (two scions grafted onto one rootstock), and inverted Y grafting (one scion grafted onto two rootstocks) at the seedling stage. K deficiency (0.03mM for standard and Y grafting, and 0.01mM for inverted Y grafting) increased the root abscisic acid (ABA) concentration by 1.6- to 3.1-fold and xylem ABA delivery rates by 1.8- to 4.6-fold. The K deficiency also decreased the delivery rates of xylem cytokinins [CKs; including the zeatin riboside (ZR) and isopentenyl adenosine (iPA) type] by 29-65% and leaf CK concentration by 16-57%. The leaf ABA concentration and xylem ABA deliveries were consistently greater in CCRI41 (more sensitive to K deficiency) than in SCRC22 (less sensitive to K deficiency) scions under K deficiency, and ZR- and iPA-type levels were consistently lower in the former than in the latter, irrespective of rootstock cultivar or grafting type, indicating that cotton shoot influences the levels of ABA and CKs in leaves and xylem sap. Because the scions had little influence on phytohormone levels in the roots (rootstocks) of all three types of grafts and rootstock xylem sap (collected below the graft union) of Y and inverted Y grafts, it appears that the site for basipetal feedback signal(s) involved in the regulation of xylem phytohormones is the hypocotyl of cotton seedlings. Also, the target of this feedback signal(s) is more likely to be the changes in xylem phytohormones within tissues of the hypocotyl rather than the export of phytohormones from the roots.
Figures


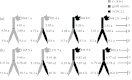



References
-
- Albacete A, Martinez-Andujar C, Ghanem ME, Acosta M, Sanchez-Bravo J, Asins MJ, Cuartero J, Lutts S, Dodd IC, Pérez-Alfocea F. 2009. Rootstock-mediated changes in xylem ionic and hormonal status are correlated with delayed leaf senescence, and increased leaf area and crop productivity in salinized tomato Plant, Cell and Environment 32 928–938 - PubMed
-
- Bednarz CW, Oosterhuis DM. 1995. Plant potassium partitioning during progression of deficiency symptoms in cotton (Gossypium hirsutum). Better Crops Potash and Phosphate Institute 79 12–14
-
- Beveridge CA, Murfet IC, Kerhoas L, Sotta B, Miginiac E, Rameau C. 1997. The shoot controls zeatin riboside export from pea roots. Evidence from the branching mutant rms4 The Plant Journal 11 339–345
-
- Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, Lin JF, Wu SH, Swidzinski J, Ishizaki K, Leaver CJ. 2005. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis The Plant Journal 42 567–585 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

