Detection of human herpesvirus 8 by quantitative polymerase chain reaction: development and standardisation of methods
- PMID: 22963082
- PMCID: PMC3490733
- DOI: 10.1186/1471-2334-12-210
Detection of human herpesvirus 8 by quantitative polymerase chain reaction: development and standardisation of methods
Abstract
Background: Human herpesvirus 8 (HHV-8), the aetiological agent of Kaposi's sarcoma (KS), multicentric Castleman's disease (MCD), and primary effusion lymphoma (PEL) is rare in Australia, but endemic in Sub-Saharan Africa, parts of South-east Asia and Oceania. While the treatment of external KS lesions can be monitored by clinical observation, the internal lesions of KS, MCD and PEL require extensive and expensive internal imaging, or autopsy. In patients with MCD and PEL, if HHV-8 viraemia is not reduced quickly, ~50% die within 24 months. HHV-8 qPCR is a valuable tool for monitoring HHV-8 viraemia, but is not available in many parts of the world, including those with high prevalence of KS and HHV-8.
Methods: A new molecular facility with stringent three-phase workflow was established, adhering to NPAAC and CLSI guidelines. Three fully validated quantitative assays were developed: two for detection and quantification of HHV-8; one for GAPDH, necessary for normalisation of viral loads in tissue and peripheral blood.
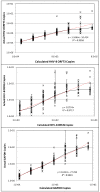
Results: The HHV-8 ORF73 and ORF26 qPCR assays were 100% specific. All qPCR assays, displayed a broad dynamic range (102 to 1010 copies/μL TE Buffer) with a limit of detection of 4.85x103, 5.61x102, and 2.59x102 copies/μL TE Buffer and a limit of quantification of 4.85x103, 3.01x102, and 1.38x102 copies/μL TE Buffer for HHV-8 ORF73, HHV-8 ORF26, and GAPDH respectively.The assays were tested on a panel of 35 KS biopsies from Queensland. All were HHV-8 qPCR positive with average viral load of 2.96x105 HHV-8 copies/μL DNA extract (range: 4.37x103 to 1.47x106 copies/μL DNA extract): When normalised these equate to an average viral load of 2.44x104 HHV-8 copies/103 cells (range: 2.20x102 to 7.38x105 HHV-8 copies/103 cells).
Conclusions: These are the first fully optimised, validated and MIQE compliant HHV-8 qPCR assays established in Australia. They worked well for qualitative detection of HHV-8 in archival tissue, and are well-suited for quantitative detection in whole blood. They are now available for research, for clinical diagnosis of HHV-8 infection, and for monitoring treatment efficacy.
Figures


References
-
- Meng YX, Spira TJ, Bhat GJ, Birch CJ, Druce JD, Edlin BR, Edwards R, Gunthel C, Newton R, Stamey FR. et al.Individuals from North America, Australasia, and Africa are infected with four different genotypes of human herpesvirus 8. Virology. 1999;261(1):106–119. doi: 10.1006/viro.1999.9853. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials

