Non-peptidergic primary afferents are presynaptic to neurokinin-1 receptor immunoreactive lamina I projection neurons in rat spinal cord
- PMID: 22963197
- PMCID: PMC3495683
- DOI: 10.1186/1744-8069-8-64
Non-peptidergic primary afferents are presynaptic to neurokinin-1 receptor immunoreactive lamina I projection neurons in rat spinal cord
Abstract
Background: Pain-related (nociceptive) information is carried from the periphery to the dorsal horn of the spinal cord mostly by two populations of small diameter primary afferents, the peptidergic and the non-peptidergic. The peptidergic population expresses neuropeptides, such as substance P and calcitonin gene-related peptide, while the non-peptidergic fibers are devoid of neuropeptides, express the purinergic receptor P2X3, and bind the isolectin B4 (IB4). Although it has been known for some time that in rat the peptidergic afferents terminate mostly in lamina I and outer lamina II and non-peptidergic afferents in inner lamina II, the extent of the termination of the latter population in lamina I was never investigated as it was considered as very minor. Because our preliminary evidence suggested otherwise, we decided to re-examine the termination of non-peptidergic afferents in lamina I, in particular with regards to their innervation of projection neurons expressing substance P receptors (NK-1r). We used retrograde labeling of neurons from the parabrachial nucleus combined with lectin IB4 binding and immunocytochemistry. Samples were examined by confocal and electron microscopy.
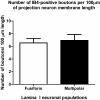
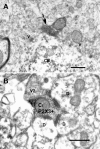
Results: By confocal microscopy, we studied the termination of non-peptidergic afferents in lamina I using IB4 binding and P2X3 immunoreactivity as markers, in relation to CGRP immunoreactivy, a marker of peptidergic afferents. The number of IB4 or P2X3-labeled fibers in lamina I was higher than previously thought, although they were less abundant than CGRP-labeled afferents. There were very few fibers double-labeled for CGRP and either P2X3 or IB4. We found a considerable number of IB4-positive fiber varicosities in close apposition to NK-1r-positive lamina I projection neurons, which were distinct from peptidergic varicosities. Furthermore, we confirmed at the ultrastructural level that there were bona fide synapses between P2X3-immunoreactive non-peptidergic boutons and neurokinin-1 receptor-positive lamina I dendrites.
Conclusions: These results indicate the presence of direct innervation by non-peptidergic nociceptive afferents of lamina I projection neurons expressing NK-1r. Further investigations are needed to better understand the role of these connections in physiological conditions and chronic pain states.
Figures






Similar articles
-
Limited changes in spinal lamina I dorsal horn neurons following the cytotoxic ablation of non-peptidergic C-fibers.Mol Pain. 2015 Sep 9;11:54. doi: 10.1186/s12990-015-0060-z. Mol Pain. 2015. PMID: 26353788 Free PMC article.
-
The majority of bladder sensory afferents to the rat lumbosacral spinal cord are both IB4- and CGRP-positive.Brain Res. 2005 Nov 16;1062(1-2):86-91. doi: 10.1016/j.brainres.2005.09.026. Epub 2005 Nov 2. Brain Res. 2005. PMID: 16263099
-
Projection neurons in lamina I of rat spinal cord with the neurokinin 1 receptor are selectively innervated by substance p-containing afferents and respond to noxious stimulation.J Neurosci. 2002 May 15;22(10):4103-13. doi: 10.1523/JNEUROSCI.22-10-04103.2002. J Neurosci. 2002. PMID: 12019329 Free PMC article.
-
Anatomy of primary afferents and projection neurones in the rat spinal dorsal horn with particular emphasis on substance P and the neurokinin 1 receptor.Exp Physiol. 2002 Mar;87(2):245-9. doi: 10.1113/eph8702351. Exp Physiol. 2002. PMID: 11856970 Review.
-
Transgenic Mouse Models for the Tracing of “Pain” Pathways.In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 7. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 7. PMID: 21882471 Free Books & Documents. Review.
Cited by
-
Spinal cord projection neurons: a superficial, and also deep, analysis.Curr Opin Physiol. 2019 Oct;11:109-115. doi: 10.1016/j.cophys.2019.10.002. Epub 2019 Oct 10. Curr Opin Physiol. 2019. PMID: 32864531 Free PMC article.
-
Neural processing of itch.Neuroscience. 2013 Oct 10;250:697-714. doi: 10.1016/j.neuroscience.2013.07.035. Epub 2013 Jul 24. Neuroscience. 2013. PMID: 23891755 Free PMC article. Review.
-
Spatial and temporal pattern of changes in the number of GAD65-immunoreactive inhibitory terminals in the rat superficial dorsal horn following peripheral nerve injury.Mol Pain. 2014 Sep 4;10:57. doi: 10.1186/1744-8069-10-57. Mol Pain. 2014. PMID: 25189404 Free PMC article.
-
Central Neuropathic Pain Development Modulation Using Coffee Extract Major Polyphenolic Compounds in Spinal-Cord-Injured Female Mice.Biology (Basel). 2022 Nov 4;11(11):1617. doi: 10.3390/biology11111617. Biology (Basel). 2022. PMID: 36358318 Free PMC article.
-
Limited changes in spinal lamina I dorsal horn neurons following the cytotoxic ablation of non-peptidergic C-fibers.Mol Pain. 2015 Sep 9;11:54. doi: 10.1186/s12990-015-0060-z. Mol Pain. 2015. PMID: 26353788 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

