A novel reproductive peptide, phoenixin
- PMID: 22963497
- PMCID: PMC3556183
- DOI: 10.1111/j.1365-2826.2012.02381.x
A novel reproductive peptide, phoenixin
Abstract
Normal anterior pituitary function is essential for fertility. Release from the gland of the reproductive hormones luteinising hormone and follicle-stimulating hormone is regulated primarily by hypothalamically-derived gonadotrophin-releasing hormone (GnRH), although other releasing factors (RF) have been postulated to exist. Using a bioinformatic approach, we have identified a novel peptide, phoenixin, that regulates pituitary gonadotrophin secretion by modulating the expression of the GnRH receptor, an action with physiologically relevant consequences. Compromise of phoenixin in vivo using small interfering RNA resulted in the delayed appearance of oestrus and a reduction in GnRH receptor expression in the pituitary. Phoenixin may represent a new class of hypothalamically-derived pituitary priming factors that sensitise the pituitary to the action of other RFs, rather than directly stimulating the fusion of secretary vesicles to pituitary membranes.
© 2012 British Society for Neuroendocrinology.
Conflict of interest statement
AUTHOR INFORMATION
The authors declare no competing financial interests. R.M.L. and J.K.C. are employees of Phoenix Pharmaceuticals.
Figures
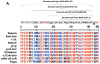




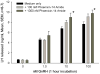









References
-
- McCann SM, Lumpkin MD, Mizunuma H, Khorram O, Samson WK. Brain peptides in control of anterior pituitary hormone secretion. Peptides. 1984;5:3–7. - PubMed
-
- Daniel PM. The blood supply of the hypothalamus and pituitary gland. Br Med Bull. 1966;22:202–208. - PubMed
-
- Gahete MD, Duran-Rado M, Luque RM, Martinez-Fuentes AJ, Quintero A, Gutierrez-Pascual E, Cordoba-Chacon J, Malagon MM, Gracia-Navarro F, Castano JP. Understanding the multifactorial control of growth hormone release by somatotropes: Lessons from comparative endocrinology. Ann NY Acad Sci. 2009;1163:137–153. - PubMed
-
- Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. Regulation of gonadotropin subunit gene transcription. J Mol Endo. 2004;33:559–584. - PubMed
-
- Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111:1737–1739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

