Chitin synthases with a myosin motor-like domain control the resistance of Aspergillus fumigatus to echinocandins
- PMID: 22964252
- PMCID: PMC3497188
- DOI: 10.1128/AAC.00752-12
Chitin synthases with a myosin motor-like domain control the resistance of Aspergillus fumigatus to echinocandins
Abstract
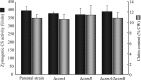
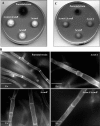
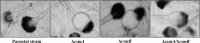
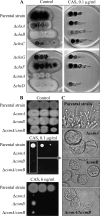
Aspergillus fumigatus has two chitin synthases (CSMA and CSMB) with a myosin motor-like domain (MMD) arranged in a head-to-head configuration. To understand the function of these chitin synthases, single and double csm mutant strains were constructed and analyzed. Although there was a slight reduction in mycelial growth of the mutants, the total chitin synthase activity and the cell wall chitin content were similar in the mycelium of all of the mutants and the parental strain. In the conidia, chitin content in the ΔcsmA strain cell wall was less than half the amount found in the parental strain. In contrast, the ΔcsmB mutant strain and, unexpectedly, the ΔcsmA/ΔcsmB mutant strain did not show any modification of chitin content in their conidial cell walls. In contrast to the hydrophobic conidia of the parental strain, conidia of all of the csm mutants were hydrophilic due to the presence of an amorphous material covering the hydrophobic surface-rodlet layer. The deletion of CSM genes also resulted in an increased susceptibility of resting and germinating conidia to echinocandins. These results show that the deletion of the CSMA and CSMB genes induced a significant disorganization of the cell wall structure, even though they contribute only weakly to the overall cell wall chitin synthesis.
Figures



References
-
- Aimanianda V, et al. 2009. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460:1117–1121 - PubMed
-
- Aufauvre-Brown A, Mellado E, Gow NA, Holden DW. 1997. Aspergillus fumigatus chsE: a gene related to CHS3 of Saccharomyces cerevisiae and important for hyphal growth and conidiophore development but not pathogenicity. Fungal Genet. Biol. 21:141–152 - PubMed
-
- Beauvais A, Drake R, Ng K, Diaquin M, Latge JP. 1993. Characterization of the 1,3-beta-glucan synthase of Aspergillus fumigatus. J. Gen. Microbiol. 139:3071–3078 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

