Mapping spatial approximations between the amino terminus of secretin and each of the extracellular loops of its receptor using cysteine trapping
- PMID: 22964305
- PMCID: PMC3509059
- DOI: 10.1096/fj.12-212399
Mapping spatial approximations between the amino terminus of secretin and each of the extracellular loops of its receptor using cysteine trapping
Abstract
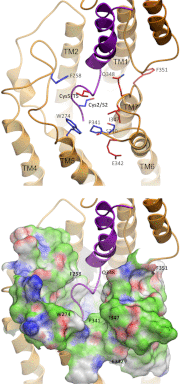
While it is evident that the carboxyl-terminal region of natural peptide ligands bind to the amino-terminal domain of class B GPCRs, how their biologically critical amino-terminal regions dock to the receptor is unclear. We utilize cysteine trapping to systematically explore spatial approximations among residues in the first five positions of secretin and in every position within the receptor extracellular loops (ECLs). Only Cys(2) and Cys(5) secretin analogues exhibited full activity and retained moderate binding affinity (IC(50): 92±4 and 83±1 nM, respectively). When these peptides probed 61 human secretin receptor cysteine-replacement mutants, a broad network of receptor residues could form disulfide bonds consistent with a dynamic ligand-receptor interface. Two distinct patterns of disulfide bond formation were observed: Cys(2) predominantly labeled residues in the amino terminus of ECL2 and ECL3 (relative labeling intensity: Ser(340), 94±7%; Pro(341), 84±9%; Phe(258), 73±5%; Trp(274) 62±8%), and Cys(5) labeled those in the carboxyl terminus of ECL2 and ECL3 (Gln(348), 100%; Ile(347), 73±12%; Glu(342), 59±10%; Phe(351), 58±11%). These constraints were utilized in molecular modeling, providing improved understanding of the structure of the transmembrane bundle and interconnecting loops, the orientation between receptor domains, and the molecular basis of ligand docking. Key spatial approximations between peptide and receptor predicted by this model (H(1)-W(274), D(3)-N(268), G(4)-F(258)) were supported by mutagenesis and residue-residue complementation studies.
Figures






References
-
- Mayo K. E., Miller L. J., Bataille D., Dalle S., Goke B., Thorens B., Drucker D. J. (2003) International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol. Rev. 55, 167–194 - PubMed
-
- Parthier C., Reedtz-Runge S., Rudolph R., Stubbs M. T. (2009) Passing the baton in class B GPCRs: peptide hormone activation via helix induction? Trends Biochem. Sci. 34, 303–310 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

