Oncogenic KRAS-induced epiregulin overexpression contributes to aggressive phenotype and is a promising therapeutic target in non-small-cell lung cancer
- PMID: 22964644
- PMCID: PMC4451140
- DOI: 10.1038/onc.2012.402
Oncogenic KRAS-induced epiregulin overexpression contributes to aggressive phenotype and is a promising therapeutic target in non-small-cell lung cancer
Abstract
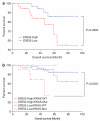
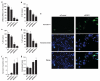
KRAS mutations are one of the most common driver mutations in non-small-cell lung cancer (NSCLC) and finding druggable target molecules to inhibit oncogenic KRAS signaling is a significant challenge in NSCLC therapy. We recently identified epiregulin (EREG) as one of several putative transcriptional targets of oncogenic KRAS signaling in both KRAS-mutant NSCLC cells and immortalized bronchial epithelial cells expressing ectopic mutant KRAS. In the current study, we found that EREG is overexpressed in NSCLCs harboring KRAS, BRAF or EGFR mutations compared with NSCLCs with wild-type KRAS/BRAF/EGFR. Small interfering RNAs (siRNAs) targeting mutant KRAS, but not an siRNA targeting wild-type KRAS, significantly reduced EREG expression in KRAS-mutant and EREG-overexpressing NSCLC cell lines. In these cell lines, EREG expression was downregulated by MEK and ERK inhibitors. Importantly, EREG expression significantly correlated with KRAS expression or KRAS copy number in KRAS-mutant NSCLC cell lines. Further expression analysis using 89 NSCLC specimens showed that EREG was predominantly expressed in NSCLCs with pleural involvement, lymphatic permeation or vascular invasion and in KRAS-mutant adenocarcinomas. In addition, multivariate analysis revealed that EREG expression is an independent prognostic marker and EREG overexpression in combination with KRAS mutations was associated with an unfavorable prognosis for lung adenocarcinoma patients. In KRAS-mutant and EREG overexpressing NSCLC cells, siRNA-mediated EREG silencing inhibited anchorage-dependent and -independent growth and induced apoptosis. Our findings suggest that oncogenic KRAS-induced EREG overexpression contributes to an aggressive phenotype and could be a promising therapeutic target in oncogenic KRAS-driven NSCLC.
Figures
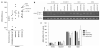
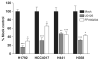


References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

