Nutritional control of growth and development in yeast
- PMID: 22964838
- PMCID: PMC3430547
- DOI: 10.1534/genetics.111.135731
Nutritional control of growth and development in yeast
Abstract
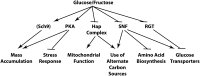
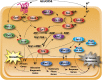
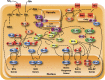
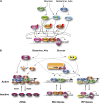
Availability of key nutrients, such as sugars, amino acids, and nitrogen compounds, dictates the developmental programs and the growth rates of yeast cells. A number of overlapping signaling networks--those centered on Ras/protein kinase A, AMP-activated kinase, and target of rapamycin complex I, for instance--inform cells on nutrient availability and influence the cells' transcriptional, translational, posttranslational, and metabolic profiles as well as their developmental decisions. Here I review our current understanding of the structures of the networks responsible for assessing the quantity and quality of carbon and nitrogen sources. I review how these signaling pathways impinge on transcriptional, metabolic, and developmental programs to optimize survival of cells under different environmental conditions. I highlight the profound knowledge we have gained on the structure of these signaling networks but also emphasize the limits of our current understanding of the dynamics of these signaling networks. Moreover, the conservation of these pathways has allowed us to extrapolate our finding with yeast to address issues of lifespan, cancer metabolism, and growth control in more complex organisms.
Figures




References
-
- Ahuatzi D., Herrero P., de la Cera T., Moreno F., 2004. The glucose-regulated nuclear localization of hexokinase 2 in Saccharomyces cerevisiae is Mig1-dependent. J. Biol. Chem. 279: 14440–14446 - PubMed
-
- Ahuatzi D., Riera A., Pelaez R., Herrero P., Moreno F., 2007. Hxk2 regulates the phosphorylation state of Mig1 and therefore its nucleocytoplasmic distribution. J. Biol. Chem. 282: 4485–4493 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

