The Ighmbp2 helicase structure reveals the molecular basis for disease-causing mutations in DMSA1
- PMID: 22965130
- PMCID: PMC3505976
- DOI: 10.1093/nar/gks792
The Ighmbp2 helicase structure reveals the molecular basis for disease-causing mutations in DMSA1
Abstract
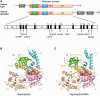
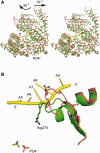
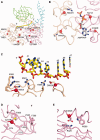
Mutations in immunoglobulin µ-binding protein 2 (Ighmbp2) cause distal spinal muscular atrophy type 1 (DSMA1), an autosomal recessive disease that is clinically characterized by distal limb weakness and respiratory distress. However, despite extensive studies, the mechanism of disease-causing mutations remains elusive. Here we report the crystal structures of the Ighmbp2 helicase core with and without bound RNA. The structures show that the overall fold of Ighmbp2 is very similar to that of Upf1, a key helicase involved in nonsense-mediated mRNA decay. Similar to Upf1, domains 1B and 1C of Ighmbp2 undergo large conformational changes in response to RNA binding, rotating 30° and 10°, respectively. The RNA binding and ATPase activities of Ighmbp2 are further enhanced by the R3H domain, located just downstream of the helicase core. Mapping of the pathogenic mutations of DSMA1 onto the helicase core structure provides a molecular basis for understanding the disease-causing consequences of Ighmbp2 mutations.
Figures



References
-
- Mellins RB. Pulmonary physiotherapy in the pediatric age group. Am. Rev. Respir. Dis. 1974;110:137–142. - PubMed
-
- Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. - PubMed
-
- Gubitz AK, Feng W, Dreyfuss G. The SMN complex. Exp. Cell Res. 2004;296:51–56. - PubMed
-
- Paushkin S, Gubitz AK, Massenet S, Dreyfuss G. The SMN complex, an assemblyosome of ribonucleoproteins. Curr. Opin. Cell Biol. 2002;14:305–312. - PubMed
-
- Meister G, Eggert C, Fischer U. SMN-mediated assembly of RNPs: a complex story. Trends Cell. Biol. 2002;12:472–478. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

