Common chaperone activity in the G-domain of trGTPase protects L11-L12 interaction on the ribosome
- PMID: 22965132
- PMCID: PMC3505967
- DOI: 10.1093/nar/gks833
Common chaperone activity in the G-domain of trGTPase protects L11-L12 interaction on the ribosome
Abstract
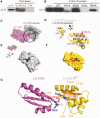
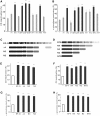
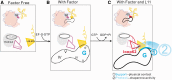
Translational GTPases (trGTPases) regulate all phases of protein synthesis. An early event in the interaction of a trGTPase with the ribosome is the contact of the G-domain with the C-terminal domain (CTD) of ribosomal protein L12 (L12-CTD) and subsequently interacts with the N-terminal domain of L11 (L11-NTD). However, the structural and functional relationships between L12-CTD and L11-NTD remain unclear. Here, we performed mutagenesis, biochemical and structural studies to identify the interactions between L11-NTD and L12-CTD. Mutagenesis of conserved residues in the interaction site revealed their role in the docking of trGTPases. During docking, loop62 of L11-NTD protrudes into a cleft in L12-CTD, leading to an open conformation of this domain and exposure of hydrophobic core. This unfavorable situation for L12-CTD stability is resolved by a chaperone-like activity of the contacting G-domain. Our results suggest that all trGTPases-regardless of their different specific functions-use a common mechanism for stabilizing the L11-NTD•L12-CTD interactions.
Figures




Similar articles
-
Ribosomal protein L7/L12 is required for GTPase translation factors EF-G, RF3, and IF2 to bind in their GTP state to 70S ribosomes.FEBS J. 2017 Jun;284(11):1631-1643. doi: 10.1111/febs.14067. Epub 2017 Apr 10. FEBS J. 2017. PMID: 28342293 Free PMC article.
-
A conserved proline switch on the ribosome facilitates the recruitment and binding of trGTPases.Nat Struct Mol Biol. 2012 Mar 11;19(4):403-10. doi: 10.1038/nsmb.2254. Nat Struct Mol Biol. 2012. PMID: 22407015
-
Complementary charge-based interaction between the ribosomal-stalk protein L7/12 and IF2 is the key to rapid subunit association.Proc Natl Acad Sci U S A. 2018 May 1;115(18):4649-4654. doi: 10.1073/pnas.1802001115. Epub 2018 Apr 23. Proc Natl Acad Sci U S A. 2018. PMID: 29686090 Free PMC article.
-
The L7/L12 ribosomal domain of the ribosome: structural and functional studies.FEBS Lett. 1997 May 5;407(3):253-6. doi: 10.1016/s0014-5793(97)00361-x. FEBS Lett. 1997. PMID: 9175862 Review.
-
Structure and function of the acidic ribosomal stalk proteins.Curr Protein Pept Sci. 2002 Feb;3(1):93-106. doi: 10.2174/1389203023380756. Curr Protein Pept Sci. 2002. PMID: 12370014 Review.
Cited by
-
EF-G and EF4: translocation and back-translocation on the bacterial ribosome.Nat Rev Microbiol. 2014 Feb;12(2):89-100. doi: 10.1038/nrmicro3176. Epub 2013 Dec 23. Nat Rev Microbiol. 2014. PMID: 24362468 Review.
-
EF4 disengages the peptidyl-tRNA CCA end and facilitates back-translocation on the 70S ribosome.Nat Struct Mol Biol. 2016 Feb;23(2):125-31. doi: 10.1038/nsmb.3160. Epub 2016 Jan 25. Nat Struct Mol Biol. 2016. PMID: 26809121
-
The GTPase BipA expressed at low temperature in Escherichia coli assists ribosome assembly and has chaperone-like activity.J Biol Chem. 2018 Nov 23;293(47):18404-18419. doi: 10.1074/jbc.RA118.002295. Epub 2018 Oct 10. J Biol Chem. 2018. PMID: 30305394 Free PMC article.
-
EF-G catalyzes tRNA translocation by disrupting interactions between decoding center and codon-anticodon duplex.Nat Struct Mol Biol. 2014 Sep;21(9):817-24. doi: 10.1038/nsmb.2869. Epub 2014 Aug 10. Nat Struct Mol Biol. 2014. PMID: 25108354
-
Ribosomal protein L7/L12 is required for GTPase translation factors EF-G, RF3, and IF2 to bind in their GTP state to 70S ribosomes.FEBS J. 2017 Jun;284(11):1631-1643. doi: 10.1111/febs.14067. Epub 2017 Apr 10. FEBS J. 2017. PMID: 28342293 Free PMC article.
References
-
- Diaconu M, Kothe U, Schlunzen F, Fischer N, Harms JM, Tonevitsky AG, Stark H, Rodnina MV, Wahl MC. Structural basis for the function of the ribosomal L7/12 stalk in factor binding and GTPase activation. Cell. 2005;121:991–1004. - PubMed
-
- Traut RR, Dey D, Bochkariov DE, Oleinikov AV, Jokhadze GG, Hamman B, Jameson D. Location and domain structure of Escherichia coli ribosomal protein L7/L12: site specific cysteine cross-linking and attachment of fluorescent probes. Biochem. Cell Biol. 1995;73:949–958. - PubMed
-
- Agrawal RK, Linde J, Sengupta J, Nierhaus KH, Frank J. Localization of L11 protein on the ribosome and elucidation of its involvement in EF-G-dependent translocation. J. Mol. Biol. 2001;311:777–787. - PubMed
-
- Kavran JM, Steitz TA. Structure of the base of the L7/L12 stalk of the Haloarcula marismortui large ribosomal subunit: analysis of L11 movements. J. Mol. Biol. 2007;371:1047–1059. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

