A hybrid G-quadruplex structure formed between RNA and DNA explains the extraordinary stability of the mitochondrial R-loop
- PMID: 22965135
- PMCID: PMC3488243
- DOI: 10.1093/nar/gks802
A hybrid G-quadruplex structure formed between RNA and DNA explains the extraordinary stability of the mitochondrial R-loop
Abstract
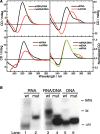
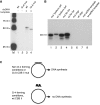
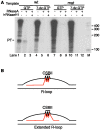
In human mitochondria the transcription machinery generates the RNA primers needed for initiation of DNA replication. A critical feature of the leading-strand origin of mitochondrial DNA replication is a CG-rich element denoted conserved sequence block II (CSB II). During transcription of CSB II, a G-quadruplex structure forms in the nascent RNA, which stimulates transcription termination and primer formation. Previous studies have shown that the newly synthesized primers form a stable and persistent RNA-DNA hybrid, a R-loop, near the leading-strand origin of DNA replication. We here demonstrate that the unusual behavior of the RNA primer is explained by the formation of a stable G-quadruplex structure, involving the CSB II region in both the nascent RNA and the non-template DNA strand. Based on our data, we suggest that G-quadruplex formation between nascent RNA and the non-template DNA strand may be a regulated event, which decides the fate of RNA primers and ultimately the rate of initiation of DNA synthesis in human mitochondria.
Figures



References
-
- Ojala D, Montoya J, Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981;290:470–474. - PubMed
-
- Clayton DA. Replication and transcription of vertebrate mitochondrial DNA. Annu. Rev. Cell Biol. 1991;7:453–478. - PubMed
-
- Falkenberg M, Larsson NG, Gustafsson CM. DNA replication and transcription in mammalian mitochondria. Annu. Rev. Biochem. 2007;76:679–699. - PubMed
-
- Falkenberg M, Gaspari M, Rantanen A, Trifunovic A, Larsson NG, Gustafsson CM. Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nat. Genet. 2002;31:289–294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

