Evaluation of changes in the tumor microenvironment after sorafenib therapy by sequential histology and 18F-fluoromisonidazole hypoxia imaging in renal cell carcinoma
- PMID: 22965141
- PMCID: PMC3583814
- DOI: 10.3892/ijo.2012.1624
Evaluation of changes in the tumor microenvironment after sorafenib therapy by sequential histology and 18F-fluoromisonidazole hypoxia imaging in renal cell carcinoma
Abstract
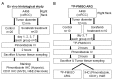
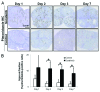
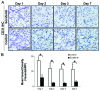
The mechanistic dissociation of 'tumor starvation' versus 'vascular normalization' following anti-angiogenic therapy is a subject of intense controversy in the field of experimental research. In addition, accurately evaluating changes of the tumor microenvironment after anti-angiogenic therapy is important for optimizing treatment strategy. Sorafenib has considerable anti-angiogenic effects that lead to tumor starvation and induce tumor hypoxia in the highly vascularized renal cell carcinoma (RCC) xenografts. 18F-fluoromisonidazole (18F‑FMISO) is a proven hypoxia imaging probe. Thus, to clarify early changes in the tumor microenvironment following anti-angiogenic therapy and whether 18F-FMISO imaging can detect those changes, we evaluated early changes in the tumor microenvironment after sorafenib treatment in an RCC xenograft by sequential histological analysis and 18F-FMISO autoradiography (ARG). A human RCC xenograft (A498) was established in nude mice, for histological studies and ARG, and further assigned to the control and sorafenib-treated groups (80 mg/kg, per os). Mice were sacrificed on Days 1, 2, 3 and 7 in the histological study, and on Days 3 and 7 in ARG after sorafenib treatment. Tumor volume was measured every day. 18F-FMISO and pimonidazole were injected intravenously 4 and 2 h before sacrifice, respectively. Tumor sections were stained with hematoxylin and eosin and immunohistochemically with pimonidazole and CD31. Intratumoral 18F-FMISO distribution was quantified in ARG. Tumor volume did not significantly change on Day 7 after sorafenib treatment. In the histological study, hypoxic fraction significantly increased on Day 2, mean vessel density significantly decreased on Day 1 and necrosis area significantly increased on Day 2 after sorafenib treatment. Intratumoral 18F-FMISO distribution significantly increased on Days 3 (10.2-fold, p<0.01) and 7 (4.1-fold, p<0.01) after sorafenib treatment. The sequential histological evaluation of the tumor microenvironment clarified tumor starvation in A498 xenografts treated with sorafenib. 18F-FMISO hypoxia imaging confirmed the tumor starvation. 18F-FMISO PET may contribute to determine an optimum treatment protocol after anti-angiogenic therapy.
Figures



Similar articles
-
Dual tracer evaluation of dynamic changes in intratumoral hypoxic and proliferative states after radiotherapy of human head and neck cancer xenografts using radiolabeled FMISO and FLT.BMC Cancer. 2014 Sep 22;14:692. doi: 10.1186/1471-2407-14-692. BMC Cancer. 2014. PMID: 25245041 Free PMC article.
-
Changes in tumor oxygen state after sorafenib therapy evaluated by 18F-fluoromisonidazole hypoxia imaging of renal cell carcinoma xenografts.Oncol Lett. 2017 Aug;14(2):2341-2346. doi: 10.3892/ol.2017.6371. Epub 2017 Jun 12. Oncol Lett. 2017. PMID: 28781672 Free PMC article.
-
Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models.Cancer Chemother Pharmacol. 2007 Apr;59(5):561-74. doi: 10.1007/s00280-006-0393-4. Epub 2006 Dec 8. Cancer Chemother Pharmacol. 2007. PMID: 17160391
-
Sorafenib and thyroid cancer.BioDrugs. 2013 Dec;27(6):615-28. doi: 10.1007/s40259-013-0049-y. BioDrugs. 2013. PMID: 23818056 Review.
-
Sorafenib in renal cell carcinoma.Pak J Pharm Sci. 2014 Jan;27(1):203-8. Pak J Pharm Sci. 2014. PMID: 24374450 Review.
Cited by
-
Combining radiotherapy with sunitinib: lessons (to be) learned.Angiogenesis. 2015 Oct;18(4):385-95. doi: 10.1007/s10456-015-9476-3. Epub 2015 Jul 23. Angiogenesis. 2015. PMID: 26202788 Free PMC article. Review.
-
Dual tracer evaluation of dynamic changes in intratumoral hypoxic and proliferative states after radiotherapy of human head and neck cancer xenografts using radiolabeled FMISO and FLT.BMC Cancer. 2014 Sep 22;14:692. doi: 10.1186/1471-2407-14-692. BMC Cancer. 2014. PMID: 25245041 Free PMC article.
-
Tumor hypoxia: a new PET imaging biomarker in clinical oncology.Int J Clin Oncol. 2016 Aug;21(4):619-625. doi: 10.1007/s10147-015-0920-6. Epub 2015 Nov 14. Int J Clin Oncol. 2016. PMID: 26577447 Review.
-
Molecular mechanisms of hypoxia in cancer.Clin Transl Imaging. 2017;5(3):225-253. doi: 10.1007/s40336-017-0231-1. Epub 2017 May 11. Clin Transl Imaging. 2017. PMID: 28596947 Free PMC article. Review.
-
Mechanisms of resistance to sorafenib and the corresponding strategies in hepatocellular carcinoma.World J Hepatol. 2013 Jul 27;5(7):345-52. doi: 10.4254/wjh.v5.i7.345. World J Hepatol. 2013. PMID: 23898367 Free PMC article.
References
-
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. - PubMed
-
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. - PubMed
-
- Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–514. - PubMed
-
- Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

