Minimizing back exchange in the hydrogen exchange-mass spectrometry experiment
- PMID: 22965280
- PMCID: PMC3515739
- DOI: 10.1007/s13361-012-0476-x
Minimizing back exchange in the hydrogen exchange-mass spectrometry experiment
Abstract
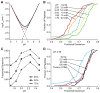
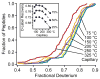
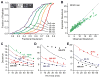
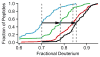
The addition of mass spectrometry (MS) analysis to the hydrogen exchange (HX) proteolytic fragmentation experiment extends powerful HX methodology to the study of large biologically important proteins. A persistent problem is the degradation of HX information due to back exchange of deuterium label during the fragmentation-separation process needed to prepare samples for MS measurement. This paper reports a systematic analysis of the factors that influence back exchange (solution pH, ionic strength, desolvation temperature, LC column interaction, flow rates, system volume). The many peptides exhibit a range of back exchange due to intrinsic amino acid HX rate differences. Accordingly, large back exchange leads to large variability in D-recovery from one residue to another as well as one peptide to another that cannot be corrected for by reference to any single peptide-level measurement. The usual effort to limit back exchange by limiting LC time provides little gain. Shortening the LC elution gradient by 3-fold only reduced back exchange by ~2%, while sacrificing S/N and peptide count. An unexpected dependence of back exchange on ionic strength as well as pH suggests a strategy in which solution conditions are changed during sample preparation. Higher salt should be used in the first stage of sample preparation (proteolysis and trapping) and lower salt (<20 mM) and pH in the second stage before electrospray injection. Adjustment of these and other factors together with recent advances in peptide fragment detection yields hundreds of peptide fragments with D-label recovery of 90% ± 5%.
Conflict of interest statement
Figures

References
-
- Konermann L, Pan J, Liu YH. Hydrogen exchange mass spectrometry for studying protein structure and dynamics. Chem Soc Rev. 2011;40:1224–1234. - PubMed
-
- Tsutsui Y, Wintrode PL. Hydrogen/deuterium, exchange-mass spectrometry: A powerful tool for probing protein structure, dynamics and interactions. Curr Med Chem. 2007;14:2344–2358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

