A parallel dual-electrode detector for capillary electrophoresis
- PMID: 22965718
- PMCID: PMC3575104
- DOI: 10.1002/elps.201200113
A parallel dual-electrode detector for capillary electrophoresis
Abstract
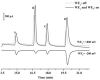
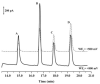
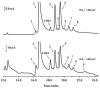
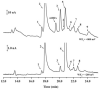
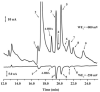
An approach to on-capillary dual-electrode detection for CE using a parallel electrode configuration has been developed. The parallel configuration provides two operating modes. In the first mode, one working electrode is held at an oxidizing potential and the second working electrode is held at a reducing potential. This results in redox cycling of analytes between the oxidized and reduced forms, enhancing sensitivity compared to single-electrode detection. In the second mode, both working electrodes are held at different oxidizing potentials. This mode provides electrochemical characterization of electrophoretic peaks. In the redox cyclying mode, signal enhancement of up to twofold was observed for the dual-electrode detection of phenolic acid standards compared to single-electrode detection. Variation in response of less than 10% from electrode to electrode was determined (at a concentration of 60 nM) indicating reproducible fabrication. LODs were determined to be as low as 5.0 nM for dual-electrode configuration. Using the dual-potential mode peak identification of targeted phenolic acids in whiskey samples were confirmed based on both migration time and current ratios.
© 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

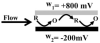
Similar articles
-
A serial dual-electrode detector based on electrogenerated bromine for capillary electrophoresis.Electrophoresis. 2014 Dec;35(24):3556-63. doi: 10.1002/elps.201400257. Epub 2014 Oct 21. Electrophoresis. 2014. PMID: 25223840
-
Sheath-flow electrochemical detection of amino acids with a copper wire electrode in capillary electrophoresis.Electrophoresis. 2012 Sep;33(17):2743-7. doi: 10.1002/elps.201200059. Electrophoresis. 2012. PMID: 22965720
-
Electrochemical detection in capillary electrophoresis with dual-parallel on-capillary electrodes.Electrophoresis. 1998 Sep;19(12):2226-32. doi: 10.1002/elps.1150191230. Electrophoresis. 1998. PMID: 9761208
-
Recent advances in electrochemical detection in capillary electrophoresis.Electrophoresis. 2000 Dec;21(18):4017-28. doi: 10.1002/1522-2683(200012)21:18<4017::AID-ELPS4017>3.0.CO;2-5. Electrophoresis. 2000. PMID: 11192121 Review.
-
A special two-working-electrode system: a summary of recent development in fabrication and application of interdigitated array electrodes.Nanotechnology. 2025 Feb 6;36(13). doi: 10.1088/1361-6528/adae15. Nanotechnology. 2025. PMID: 39854774 Review.
Cited by
-
Evaluation of dual electrode configurations for microchip electrophoresis used for voltammetric characterization of electroactive species.Analyst. 2020 Feb 3;145(3):865-872. doi: 10.1039/c9an02112d. Analyst. 2020. PMID: 31820743 Free PMC article.
References
-
- Weng Q, Xu G, Yuan K, Tang P. J. Chromatogr., B Anal. Technol. Biomed. Life Sci. 2006;835:55–61. - PubMed
-
- Xu J-J, Wang A-J, Chen H-Y. TrAC, Trends Anal. Chem. 2007;26:125–132.
-
- Du Y, Wang E. J. Sep. Sci. 2007;30:875–890. - PubMed
-
- Kong Y, Chen H, Wang Y, Soper SA. Electrophoresis. 2006;27:2940–2950. - PubMed
-
- Lunte SM, Martin RS, Lunte CE. Electroanal. Methods Biol. Mater. Marcel Dekker Inc.; New York: 2002. pp. 461–490.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

