Generation of a germ cell-specific mouse transgenic CHERRY reporter, Sohlh1-mCherryFlag
- PMID: 22965810
- PMCID: PMC3547139
- DOI: 10.1002/dvg.22347
Generation of a germ cell-specific mouse transgenic CHERRY reporter, Sohlh1-mCherryFlag
Abstract
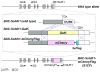
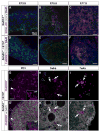
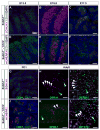
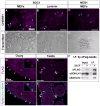
Visualization of differentiating germ cells is critical to understanding the formation of primordial follicles in the ovary, and the commitment of spermatogonial stem cells to differentiation. We engineered and generated a BAC transgenic mouse line, Sohlh1-mCherryFlag (S1CF), under the direction of the native Sohlh1 promoter. Sohlh1 is a germ cell-specific gene that encodes the basic helix-loop-helix (bHLH) transcriptional regulator that is essential in oogenesis and spermatogenesis. Sohlh1 expression is unique, and is limited to perinatal and early follicle oocytes and differentiating spermatogonia. The Sohlh1-mCherryFlag transgene was engineered to fuse SOHLH1 to the red fluorescent protein CHERRY with 3-tandem-FLAG tags. S1CF animals fluoresce specifically in the oocytes of perinatal ovaries and small follicles in adult ovaries, as well as in spermatogonia, a pattern that is similar to endogenous SOHLH1. Moreover, S1CF rescued germ cell loss and infertility in both male and female Sohlh1(-/-) animals. The FLAG-tag on S1CF was effective for immunostaining and immunoprecipitation. The Sohlh1-mCherryFlag transgenic mouse provides a unique model to study early germ cell differentiation, as well as in vivo imaging and purification of differentiating germ cells.
Copyright © 2012 Wiley Periodicals, Inc.
Figures

Similar articles
-
Auto-regulation of the Sohlh1 gene by the SOHLH2/SOHLH1/SP1 complex: implications for early spermatogenesis and oogenesis.PLoS One. 2014 Jul 8;9(7):e101681. doi: 10.1371/journal.pone.0101681. eCollection 2014. PLoS One. 2014. PMID: 25003626 Free PMC article.
-
Sohlh2 affects differentiation of KIT positive oocytes and spermatogonia.Dev Biol. 2009 Jan 1;325(1):238-48. doi: 10.1016/j.ydbio.2008.10.019. Epub 2008 Oct 29. Dev Biol. 2009. PMID: 19014927
-
Transcription factors SOHLH1 and SOHLH2 coordinate oocyte differentiation without affecting meiosis I.J Clin Invest. 2017 Jun 1;127(6):2106-2117. doi: 10.1172/JCI90281. Epub 2017 May 15. J Clin Invest. 2017. PMID: 28504655 Free PMC article.
-
Oogenesis: transcriptional regulators and mouse models.Mol Cell Endocrinol. 2012 Jun 5;356(1-2):31-9. doi: 10.1016/j.mce.2011.07.049. Epub 2011 Aug 12. Mol Cell Endocrinol. 2012. PMID: 21856374 Review.
-
Transcriptional control of KIT gene expression during germ cell development.Int J Dev Biol. 2013;57(2-4):179-84. doi: 10.1387/ijdb.130014pr. Int J Dev Biol. 2013. PMID: 23784828 Review.
Cited by
-
Developmental underpinnings of spermatogonial stem cell establishment.Andrology. 2020 Jul;8(4):852-861. doi: 10.1111/andr.12810. Epub 2020 May 24. Andrology. 2020. PMID: 32356598 Free PMC article. Review.
-
DDX4-EGFP transgenic rat model for the study of germline development and spermatogenesis.Biol Reprod. 2017 Mar 1;96(3):707-719. doi: 10.1095/biolreprod.116.142828. Biol Reprod. 2017. PMID: 28339678 Free PMC article.
-
Activation of Notch Signaling by Oocytes and Jag1 in Mouse Ovarian Granulosa Cells.Endocrinology. 2019 Dec 1;160(12):2863-2876. doi: 10.1210/en.2019-00564. Endocrinology. 2019. PMID: 31609444 Free PMC article.
-
Identification of a β-galactosidase transgene that provides a live-cell marker of transcriptional activity in growing oocytes and embryos.Mol Hum Reprod. 2015 Jul;21(7):583-93. doi: 10.1093/molehr/gav020. Epub 2015 Apr 16. Mol Hum Reprod. 2015. PMID: 25882542 Free PMC article.
-
Geography of follicle formation in the embryonic mouse ovary impacts activation pattern during the first wave of folliculogenesis.Biol Reprod. 2015 Oct;93(4):88. doi: 10.1095/biolreprod.115.131227. Epub 2015 Aug 5. Biol Reprod. 2015. PMID: 26246221 Free PMC article.
References
-
- Baibakov B, Gauthier L, Talbot P, Rankin TL, Dean J. Sperm binding to the zona pellucida is not sufficient to induce acrosome exocytosis. Development. 2007;134:933–43. - PubMed
-
- Ballow D, Meistrich ML, Matzuk M, Rajkovic A. Sohlh1 is essential for spermatogonial differentiation. Developmental biology. 2006;294:161–7. - PubMed
-
- Dann CT, Alvarado AL, Molyneux LA, Denard BS, Garbers DL, Porteus MH. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells. 2008;26:2928–37. - PubMed
-
- Falciatori I, Lillard-Wetherell K, Wu Z, Hamra FK, Garbers DL. Deriving mouse spermatogonial stem cell lines. Methods Mol Biol. 2008;450:181–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

