Synergistic anticancer effects of arsenic trioxide with bortezomib in mantle cell lymphoma
- PMID: 22965904
- PMCID: PMC3894928
- DOI: 10.1002/ajh.23317
Synergistic anticancer effects of arsenic trioxide with bortezomib in mantle cell lymphoma
Abstract
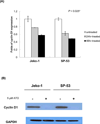
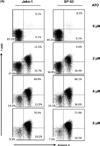
Mantle cell lymphoma (MCL) is a subtype of B-cell Non-Hodgkin's Lymphoma (NHL) and accounts for ~6% of all lymphomas. MCL is highly refractory to most chemotherapy including newer antibody-based therapeutic approaches, and high-grade MCL has one of the worst survival rates among NHLs. Therefore, the development of new therapeutic strategies to overcome drug resistance of MCL is important. In this article, we tested the effects of arsenic trioxide (As(2) O(3) , ATO) in bortezomib-resistant MCL. ATO is reported to induce complete remission in the patients with relapsed or refractory acute promyelocytic leukemia. Their effects in MCL, however, have not been explored. In this report, we show that ATO effectively inhibited the growth of MCL cells in vitro. ATO treatment also reduced cyclin D1 expression which is a genetic hallmark of MCL and NF-kB expression which was reported to have a prosurvival role in some MCL cells. The induction of apoptosis in MCL was partially due to reduced levels of cyclin D1 and increased levels of apoptosis-related molecules. The antiproliferative effects of bortezomib on MCL greatly increased when the cells were also treated with ATO, indicating ATO can sensitize MCL to bortezomib. Similar results were noted in bortezomib-resistant cell lines. In conclusion, ATO may be an alternative drug for use in combined adjuvant therapies for MCL, and further clinical testing should be performed.
Copyright © 2012 Wiley Periodicals, Inc.
Figures










Similar articles
-
Effects of arsenic trioxide alone and in combination with bortezomib in multiple myeloma RPMI 8266 cells.Asian Pac J Cancer Prev. 2013;14(11):6469-73. doi: 10.7314/apjcp.2013.14.11.6469. Asian Pac J Cancer Prev. 2013. PMID: 24377552
-
Degrasyn potentiates the antitumor effects of bortezomib in mantle cell lymphoma cells in vitro and in vivo: therapeutic implications.Mol Cancer Ther. 2010 Jul;9(7):2026-36. doi: 10.1158/1535-7163.MCT-10-0238. Epub 2010 Jul 6. Mol Cancer Ther. 2010. PMID: 20606045 Free PMC article.
-
Arsenic trioxide rewires mantle cell lymphoma response to bortezomib.Cancer Med. 2015 Nov;4(11):1754-66. doi: 10.1002/cam4.511. Epub 2015 Aug 26. Cancer Med. 2015. PMID: 26310857 Free PMC article.
-
Proteasome inhibition with bortezomib: a new therapeutic strategy for non-Hodgkin's lymphoma.Int J Cancer. 2006 Sep 1;119(5):971-9. doi: 10.1002/ijc.21805. Int J Cancer. 2006. PMID: 16557600 Review.
-
Proteasome inhibitors in mantle cell lymphoma.Best Pract Res Clin Haematol. 2012 Jun;25(2):133-41. doi: 10.1016/j.beha.2012.04.007. Epub 2012 May 16. Best Pract Res Clin Haematol. 2012. PMID: 22687449 Free PMC article. Review.
Cited by
-
TG2 and NF-κB Signaling Coordinates the Survival of Mantle Cell Lymphoma Cells via IL6-Mediated Autophagy.Cancer Res. 2016 Nov 1;76(21):6410-6423. doi: 10.1158/0008-5472.CAN-16-0595. Epub 2016 Aug 3. Cancer Res. 2016. PMID: 27488529 Free PMC article.
-
A phase II study of arsenic trioxide in patients with relapsed or refractory malignant lymphoma.Med Oncol. 2015 Mar;32(3):79. doi: 10.1007/s12032-015-0526-x. Epub 2015 Feb 20. Med Oncol. 2015. PMID: 25698531 Clinical Trial.
-
Effects of arsenic sulfide (As2S2) on B and T lymphoma cell lines and possible underlying mechanisms.Biomed Rep. 2013 Jul;1(4):583-588. doi: 10.3892/br.2013.119. Epub 2013 May 30. Biomed Rep. 2013. PMID: 24648990 Free PMC article.
-
3-Methyladenine but not antioxidants to overcome BACH2-mediated bortezomib resistance in mantle cell lymphoma.Cancer Cell Int. 2021 May 26;21(1):279. doi: 10.1186/s12935-021-01980-2. Cancer Cell Int. 2021. PMID: 34039348 Free PMC article.
-
The Development and Clinical Applications of Oral Arsenic Trioxide for Acute Promyelocytic Leukaemia and Other Diseases.Pharmaceutics. 2022 Sep 14;14(9):1945. doi: 10.3390/pharmaceutics14091945. Pharmaceutics. 2022. PMID: 36145693 Free PMC article. Review.
References
-
- Salaverria I, Perez-Galan P, Colomer D, Campo E. Mantle cell lymphoma: from pathology and molecular pathogenesis to new therapeutic perspectives. Haematologica. 2006;91:11–16. - PubMed
-
- Bonati A, Rizzoli V, Lunghi P. Arsenic trioxide in hematological malignancies:The new discovery of an ancient drug. Curr Pharm Biotechnol. 2006;7:397–405. - PubMed
-
- Douer D, Tallman MS. Arsenic trixoide:New clinical experience with and old medication in hematological melignancies. J. Clin. Oncol. 2005;33:2396–2410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
