Plasmin and chymotrypsin have distinct preferences for channel activating cleavage sites in the γ subunit of the human epithelial sodium channel
- PMID: 22966015
- PMCID: PMC3457690
- DOI: 10.1085/jgp.201110763
Plasmin and chymotrypsin have distinct preferences for channel activating cleavage sites in the γ subunit of the human epithelial sodium channel
Abstract
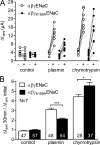
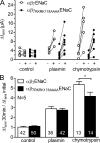
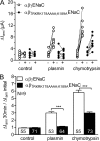
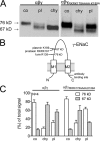
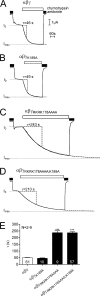
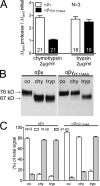
Proteolytic activation of the epithelial sodium channel (ENaC) involves cleavage of its γ subunit in a critical region targeted by several proteases. Our aim was to identify cleavage sites in this region that are functionally important for activation of human ENaC by plasmin and chymotrypsin. Sequence alignment revealed a putative plasmin cleavage site in human γENaC (K189) that corresponds to a plasmin cleavage site (K194) in mouse γENaC. We mutated this site to alanine (K189A) and expressed human wild-type (wt) αβγENaC and αβγ(K189A)ENaC in Xenopus laevis oocytes. The γ(K189A) mutation reduced but did not abolish activation of ENaC whole cell currents by plasmin. Mutating a putative prostasin site (γ(RKRK178AAAA)) had no effect on the stimulatory response to plasmin. In contrast, a double mutation (γ(RKRK178AAAA;K189A)) prevented the stimulatory effect of plasmin. We conclude that in addition to the preferential plasmin cleavage site K189, the putative prostasin cleavage site RKRK178 may serve as an alternative site for proteolytic channel activation by plasmin. Interestingly, the double mutation delayed but did not abolish ENaC activation by chymotrypsin. The time-dependent appearance of cleavage products at the cell surface nicely correlated with the stimulatory effect of chymotrypsin on ENaC currents in oocytes expressing wt or double mutant ENaC. Delayed proteolytic activation of the double mutant channel with a stepwise recruitment of so-called near-silent channels was confirmed in single-channel recordings from outside-out patches. Mutating two phenylalanines (FF174) in the vicinity of the prostasin cleavage site prevented proteolytic activation by chymotrypsin. This indicates that chymotrypsin preferentially cleaves at FF174. The close proximity of FF174 to the prostasin site may explain why mutating the prostasin site impedes channel activation by chymotrypsin. In conclusion, this study supports the concept that different proteases have distinct preferences for certain cleavage sites in γENaC, which may be relevant for tissue-specific proteolytic ENaC activation.
Figures





References
-
- Bruns J.B., Carattino M.D., Sheng S., Maarouf A.B., Weisz O.A., Pilewski J.M., Hughey R.P., Kleyman T.R. 2007. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the γ-subunit. J. Biol. Chem. 282:6153–6160 10.1074/jbc.M610636200 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

