Personalized medicine in a phase I clinical trials program: the MD Anderson Cancer Center initiative
- PMID: 22966018
- PMCID: PMC4454458
- DOI: 10.1158/1078-0432.CCR-12-1627
Personalized medicine in a phase I clinical trials program: the MD Anderson Cancer Center initiative
Abstract
Purpose: We initiated a personalized medicine program in the context of early clinical trials, using targeted agents matched with tumor molecular aberrations. Herein, we report our observations.
Patient and methods: Patients with advanced cancer were treated in the Clinical Center for Targeted Therapy. Molecular analysis was conducted in the MD Anderson Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory. Patients whose tumors had an aberration were treated with matched targeted therapy, when available. Treatment assignment was not randomized. The clinical outcomes of patients with molecular aberrations treated with matched targeted therapy were compared with those of consecutive patients who were not treated with matched targeted therapy.
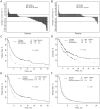
Results: Of 1,144 patients analyzed, 460 (40.2%) had 1 or more aberration. In patients with 1 molecular aberration, matched therapy (n = 175) compared with treatment without matching (n = 116) was associated with a higher overall response rate (27% vs. 5%; P < 0.0001), longer time-to-treatment failure (TTF; median, 5.2 vs. 2.2 months; P < 0.0001), and longer survival (median, 13.4 vs. 9.0 months; P = 0.017). Matched targeted therapy was associated with longer TTF compared with their prior systemic therapy in patients with 1 mutation (5.2 vs. 3.1 months, respectively; P < 0.0001). In multivariate analysis in patients with 1 molecular aberration, matched therapy was an independent factor predicting response (P = 0.001) and TTF (P = 0.0001).
Conclusion: Keeping in mind that the study was not randomized and patients had diverse tumor types and a median of 5 prior therapies, our results suggest that identifying specific molecular abnormalities and choosing therapy based on these abnormalities is relevant in phase I clinical trials.
©2012 AACR.
Figures

References
-
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. - PubMed
-
- Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CAgene in human cancers. Science. 2004;304:554. - PubMed
-
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. - PubMed
-
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

